Volume 19, Issue 4 (Jul-Aug 2025)
mljgoums 2025, 19(4): 35-40 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Raeisi M, Hooshmand F, Gheraati M, Aman Mohammadi M, Mehdinejad N. Comparative analysis of antibacterial activity and chemical composition of essential oils from Salix aegyptiaca male inflorescences and leaves. mljgoums 2025; 19 (4) :35-40
URL: http://mlj.goums.ac.ir/article-1-1920-en.html
URL: http://mlj.goums.ac.ir/article-1-1920-en.html
Mojtaba Raeisi1 

 , Fatemeh Hooshmand2
, Fatemeh Hooshmand2 

 , Marziyeh Gheraati3
, Marziyeh Gheraati3 

 , Masood Aman Mohammadi4
, Masood Aman Mohammadi4 

 , Negin Mehdinejad5
, Negin Mehdinejad5 




 , Fatemeh Hooshmand2
, Fatemeh Hooshmand2 

 , Marziyeh Gheraati3
, Marziyeh Gheraati3 

 , Masood Aman Mohammadi4
, Masood Aman Mohammadi4 

 , Negin Mehdinejad5
, Negin Mehdinejad5 


1- Food, Drug and Natural Products Health Research Center, Golestan University of Medical Sciences, Gorgan, Iran , m.raeisi2000@gmail.com
2- School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Department of Traditional Medicine, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4- Food and Beverages Safety Research Center, Urmia University of Medical Sciences, Urmia, Iran
5- Faculty of Veterinary Sciences, Islamic Azad University, Sciences and Research Branch, Tehran, Iran
2- School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Department of Traditional Medicine, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4- Food and Beverages Safety Research Center, Urmia University of Medical Sciences, Urmia, Iran
5- Faculty of Veterinary Sciences, Islamic Azad University, Sciences and Research Branch, Tehran, Iran
Keywords: Salix aegyptiaca, Essential oil, Antibacterial activity, Foodborne pathogens, Natural preservative, Male inflorescence
Full-Text [PDF 486 kb]
(663 Downloads)
| Abstract (HTML) (3214 Views)
Full-Text: (479 Views)
Introduction
Foodborne illnesses pose a persistent and significant threat to global public health. Bacterial pathogens, such as Salmonella enteritidis (S. enteritidis), Escherichia coli (E. coli), Listeria monocytogenes (L. monocytogenes), and Staphylococcus aureus (S. aureus) are major causative agents, leading to millions of infections and substantial economic burdens annually. The transmission of these bacteria most often occurs through the consumption of contaminated food products. The traditional approach to controlling these bacterial pathogens has relied on the use of synthetic preservatives and antibiotics (1). However, the rising prevalence of antibiotic-resistant bacterial strains has diminished the efficacy of these methods. Consequently, there is a growing need to find natural antimicrobial agents that can provide safer and more sustainable alternatives. Scientific interest in natural plant extracts, especially essential oils, has surged. This is primarily due to their strong antimicrobial, antioxidant, and preservative properties. Essential oils are intricate blends of volatile compounds synthesized by plants as secondary metabolites (2). These oils function as a natural defense system against pathogens, pests, and environmental stressors, which gives them an intrinsic antimicrobial ability that can be leveraged for food preservation. While the potent antibacterial properties of essential oils from plants like thyme, oregano, and clove have been well-documented, the potential of many other essential oils, including those extracted from traditional medicinal plants, has not yet been thoroughly investigated for their use against foodborne pathogens.
The medicinal properties of Salix aegyptiaca (S. aegyptiaca), commonly known as Musk Willow, are well-documented. This plant has a long history of use in traditional herbal medicine, especially in the Middle East and Central Asia. It is recognized for its anti-inflammatory, analgesic, and antioxidant effects and has traditionally been used to address a range of conditions, including headaches, digestive issues, and respiratory illnesses (3,4). The male inflorescences of S. aegyptiaca are highly prized and have a long history of traditional use for its fragrant qualities and medicinal applications, with its essential oil often being extracted (5). Traditionally, the leaves of S. aegyptiaca have been utilized for their medicinal properties, specifically for treating wounds and reducing fever (3). Prior studies have shown that different parts of S. aegyptiaca contain a variety of bioactive compounds, such as phenolic compounds, flavonoids, and tannins. These compounds are known to possess a wide range of pharmacological properties, including antimicrobial effects (5,6). Previous research on various Salix species has shown significant antimicrobial effects, suggesting the potential of this genus as a source of natural antimicrobial agents (7). Building on this evidence, our study aims to address a gap in the literature by examining the antibacterial activity of S. aegyptiaca essential oil against multiple key foodborne pathogens that are frequently linked to food spoilage and foodborne infections, such as S. aureus, E. coli, L. monocytogenes, S. enteritidis, and Pseudomonas aeruginosa (P. aeruginosa). In this study, we aim to validate the potential of S. aegyptiaca essential oil as a natural food preservative. We will achieve this by evaluating its efficacy against a range of microorganisms using several standard antimicrobial assays, including minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), agar disk diffusion, and agar well diffusion (8-11). Considering the growing consumer preference for natural and safe food options, investigating the preservative properties of S. aegyptiaca essential oil is a timely and pertinent endeavor. The results of this study have the potential to pave the way for developing this essential oil into a sustainable and effective alternative to synthetic food preservatives. This would not only improve food safety but also decrease dependence on conventional antibiotics.
Methods
Plant material collection and identification
Fresh leaves and male inflorescences of S. aegyptiaca were gathered during the early spring flowering season from Iran's Zagros Mountains to maximize their phytochemical concentration. The plant specimens were botanically identified by agricultural specialists at Gorgan University of Agricultural Sciences and Natural Resources. Following collection, the plant materials were meticulously cleaned and air-dried at ambient temperature in a shaded, well-ventilated space to prevent the degradation of volatile compounds.
Essential oil extraction
Essential oils were obtained from both male inflorescences and leaves of the plant using hydrodistillation. The extraction process was conducted with a Clevenger-type apparatus, adhering to the standards outlined in the European Pharmacopoeia. For each separate extraction, 100 grams of dried plant matter (Either male inflorescences or leaves) were combined with 1.5 liters of distilled water in a 2-liter round-bottom flask. The mixture was subjected to heating, and the resulting steam, laden with volatile oils, was subsequently condensed and collected. To ensure optimal recovery of the essential oils, the extraction process was sustained for a period of 4 hours. Given that the male inflorescences of this particular plant species contain aromatic compounds primarily during the plant’s fresh flowering stage, the extraction was executed immediately following the collection of fresh plant material to facilitate the capture of its volatile constituents. The essential oils were then isolated from the aqueous layer. Subsequently, they were dried using anhydrous sodium sulfate, filtered, and then placed in dark glass vials. To inhibit oxidative degradation, the samples were stored at 4°C. The essential oil yield was determined by calculating the ratio of the oils' weight to the dry weight of the plant material, expressed as a percentage (12).
Chemical analysis of essential oils
The chemical composition of the essential oils was determined through gas chromatography-mass spectrometry (GC-MS). The analysis was performed on a GC-MS system equipped with a fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Helium served as the carrier gas, maintaining a constant flow rate of 1 mL/min. The GC oven temperature was programmed to rise from 60°C to 240°C at a rate of 3°C/min, while the injector temperature was maintained at 250°C (13).
Bacterial strains and preparation
The antibacterial properties of the essential oils were evaluated against a panel of ten bacterial strains. These strains, which are known to be responsible for either foodborne illnesses or spoilage, included S. aureus (Persian Type Culture Collection [PTCC] 1917), E. coli (PTCC 1338), L. monocytogenes (PTCC 1783), P. aeruginosa (PTCC 1310), S. enteritidis (PTCC 1787), Shigella dysenteriae (S. dysenteriae) (PTCC 1188), Klebsiella pneumoniae (K. pneumoniae) (PTCC 1053), Alcaligenes faecalis (A. faecalis) (PTCC 1624), Serratia marcescens (S. marcescens) (PTCC 1621), and Streptococcus pyogenes (S. pyogenes) (PTCC 1762). All strains were procured from the PTCC. For each strain, a bacterial suspension was prepared by culturing it in brain-heart infusion (BHI) broth at 37°C for 18 hours to ensure it reached the exponential growth phase. The final bacterial concentration was standardized to approximately 10^6 colony-forming units (CFU)/mL by measuring the optical density at 600 nm with a spectrophotometer. This standardized inoculum was subsequently utilized in all downstream assays.
Antibacterial assays
Minimum inhibitory concentration and minimum bactericidal concentration
The MIC and MBC of the essential oils were established via the broth microdilution method. Serial twofold dilutions of the oils were prepared in 96-well microtiter plates, each containing 100 µL of BHI broth. After inoculating each well with 10 µL of a bacterial suspension adjusted to a concentration of 10^6 CFU/mL, the plates were incubated for 24 hours at 37°C. The MIC was determined as the lowest concentration of essential oil that totally prevented the visible growth of bacteria. To establish the MBC, samples from the wells with no visible growth were subcultured on nutrient agar. After 24 hours of incubation, the MBC was identified as the lowest concentration of essential oil that resulted in a 99.9% reduction in bacterial viability. All experiments were conducted in triplicate, and the mean values were reported (14).
Agar disk diffusion assay
The antibacterial efficacy of the essential oils was evaluated using the Clinical and Laboratory Standards Institute (CLSI) agar disk diffusion method. Overnight bacterial cultures were diluted in sterile saline to match a 0.5 McFarland standard, which corresponds to a density of approximately 10^6 CFU/mL. The bacterial suspension was uniformly distributed over Mueller-Hinton agar plates using a sterile cotton swab. Sterile, 6 mm paper disks, impregnated with 10 µL of essential oil (Diluted in dimethyl sulfoxide [DMSO]), were then placed on the inoculated surface. Following a 24-hour incubation at 37°C, the resulting zones of inhibition were measured with a digital caliper. For this study, gentamicin (10 µg/disc) and chloramphenicol (30 µg/disc) were utilized as positive controls, while disks containing only DMSO functioned as the negative control. All experiments were conducted in triplicate, and the final results are presented as the mean ± standard deviation (15).
Agar well diffusion assay
The agar well diffusion method was used to assess the antibacterial properties of the essential oils. First, holes measuring 6 mm in diameter were created in Mueller-Hinton agar plates that had already been seeded with bacterial suspensions. Next, 50 µL of various concentrations of essential oils (Diluted in DMSO) were added to each well. After incubating the plates at 37°C for 24 hours, the inhibition zones surrounding the wells were measured to determine the antibacterial effect. Positive and negative controls were incorporated into the experiment, similar to the disk diffusion assay. This technique enabled the evaluation of the antibacterial activity of different essential oil concentrations (16).
Statistical analysis
The antibacterial assay data were analyzed using SPSS software to evaluate the essential oils' efficacy against various bacterial strains. Prior to analysis, the Kolmogorov-Smirnov test was employed to confirm data normality, and Levene's test was used to verify homogeneity of variances. To determine statistically significant differences among the groups, a one-way analysis of variance (ANOVA) was performed, followed by Tukey's post hoc test. The level of significance was established at p < 0.05. All results were presented as the mean ± standard deviation from three independent experiments.
Results
This study’s findings reveal the chemical composition of S. aegyptiaca essential oils derived from the leaves and male inflorescences. The research also details the oils' antibacterial efficacy against several foodborne pathogens. These results are reported through an analysis of the chemical profile, MIC and MBC values, and the zones of inhibition recorded in both agar disk and well diffusion assays.
Chemical composition of salix aegyptiaca essential oils
GC-MS analysis of the essential oils extracted from both the leaves and male inflorescences revealed a variety of bioactive compounds. According to Table 1, the main compounds in the leaf oil were 1,4-dimethoxybenzene (34.78%), citronellol (13.53%), and eugenol (5.29%). In contrast, the male inflorescence oil was predominantly composed of 1,4-dimethoxybenzene (28.46%), followed by citronellol (10.75%) and carvone (5.12%). The antimicrobial properties of these compounds likely account for the efficacy of the oils against the bacterial strains examined in this study. The observed variations in the chemical composition of the oils derived from the leaves versus the male inflorescences indicate that the biological activity of the essential oils may be dependent on their specific source within the plant (17,18).
Minimum inhibitory concentration and minimum bactericidal concentration results
Table 2 summarizes the MIC and MBC values for each bacterial strain. These values indicate the specific oil concentrations required to inhibit the growth of, and to kill each strain, respectively.
Leaf essential oil
The MIC values for the leaf oil demonstrated a range from 1250 µg/mL against S. aureus to 5000 µg/mL for both S. enteritidis and S. dysenteriae. Similarly, the MBC values followed a comparable pattern, with the lowest concentration (2500 µg/mL) required to inhibit S. aureus, while S. enteritidis and S. dysenteriae needed a higher concentration of 5000 µg/mL for bactericidal effects. These findings suggest that S. aureus exhibits the highest susceptibility to the leaf oil, whereas S. enteritidis and S. dysenteriae are considerably more resistant.
Male inflorescence essential oil
The MIC and MBC values for the male inflorescence oil were consistently higher than those for the leaf oil, suggesting a diminished overall antimicrobial efficacy. The oil demonstrated its most potent inhibitory effect against S. marcescens and S. aureus, for which the lowest MIC of 2500 µg/mL was recorded. In contrast, the highest MIC of 5000 µg/mL was required to inhibit the growth of E. coli, S. enteritidis, and S. dysenteriae. For bactericidal activity, the MBC for S. marcescens and S. aureus was also at its lowest, at 2500 µg/mL. However, a significantly higher concentration of 10,000 µg/mL was necessary to achieve a bactericidal effect against S. enteritidis.
Agar disk diffusion assay
Inhibition zones, which varied by bacterial strain and essential oil type, were observed in the agar disk diffusion assay (Table 3). The leaf oil consistently produced larger inhibition zones compared to the male inflorescence oil.
Leaf oil
The extract exhibited its most potent antibacterial activity against S. aureus, as evidenced by the largest mean inhibition zone diameter of 9.38 ± 0.15 mm. Conversely, E. coli displayed the least susceptibility to the extract, with the smallest observed inhibition zone of 7.59 ± 0.20 mm. The other tested strains, including P. aeruginosa and S. marcescens, demonstrated moderate sensitivity, producing inhibition zones of 9.28 ± 0.15 mm and 9.12 ± 0.15 mm, respectively.
Foodborne illnesses pose a persistent and significant threat to global public health. Bacterial pathogens, such as Salmonella enteritidis (S. enteritidis), Escherichia coli (E. coli), Listeria monocytogenes (L. monocytogenes), and Staphylococcus aureus (S. aureus) are major causative agents, leading to millions of infections and substantial economic burdens annually. The transmission of these bacteria most often occurs through the consumption of contaminated food products. The traditional approach to controlling these bacterial pathogens has relied on the use of synthetic preservatives and antibiotics (1). However, the rising prevalence of antibiotic-resistant bacterial strains has diminished the efficacy of these methods. Consequently, there is a growing need to find natural antimicrobial agents that can provide safer and more sustainable alternatives. Scientific interest in natural plant extracts, especially essential oils, has surged. This is primarily due to their strong antimicrobial, antioxidant, and preservative properties. Essential oils are intricate blends of volatile compounds synthesized by plants as secondary metabolites (2). These oils function as a natural defense system against pathogens, pests, and environmental stressors, which gives them an intrinsic antimicrobial ability that can be leveraged for food preservation. While the potent antibacterial properties of essential oils from plants like thyme, oregano, and clove have been well-documented, the potential of many other essential oils, including those extracted from traditional medicinal plants, has not yet been thoroughly investigated for their use against foodborne pathogens.
The medicinal properties of Salix aegyptiaca (S. aegyptiaca), commonly known as Musk Willow, are well-documented. This plant has a long history of use in traditional herbal medicine, especially in the Middle East and Central Asia. It is recognized for its anti-inflammatory, analgesic, and antioxidant effects and has traditionally been used to address a range of conditions, including headaches, digestive issues, and respiratory illnesses (3,4). The male inflorescences of S. aegyptiaca are highly prized and have a long history of traditional use for its fragrant qualities and medicinal applications, with its essential oil often being extracted (5). Traditionally, the leaves of S. aegyptiaca have been utilized for their medicinal properties, specifically for treating wounds and reducing fever (3). Prior studies have shown that different parts of S. aegyptiaca contain a variety of bioactive compounds, such as phenolic compounds, flavonoids, and tannins. These compounds are known to possess a wide range of pharmacological properties, including antimicrobial effects (5,6). Previous research on various Salix species has shown significant antimicrobial effects, suggesting the potential of this genus as a source of natural antimicrobial agents (7). Building on this evidence, our study aims to address a gap in the literature by examining the antibacterial activity of S. aegyptiaca essential oil against multiple key foodborne pathogens that are frequently linked to food spoilage and foodborne infections, such as S. aureus, E. coli, L. monocytogenes, S. enteritidis, and Pseudomonas aeruginosa (P. aeruginosa). In this study, we aim to validate the potential of S. aegyptiaca essential oil as a natural food preservative. We will achieve this by evaluating its efficacy against a range of microorganisms using several standard antimicrobial assays, including minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), agar disk diffusion, and agar well diffusion (8-11). Considering the growing consumer preference for natural and safe food options, investigating the preservative properties of S. aegyptiaca essential oil is a timely and pertinent endeavor. The results of this study have the potential to pave the way for developing this essential oil into a sustainable and effective alternative to synthetic food preservatives. This would not only improve food safety but also decrease dependence on conventional antibiotics.
Methods
Plant material collection and identification
Fresh leaves and male inflorescences of S. aegyptiaca were gathered during the early spring flowering season from Iran's Zagros Mountains to maximize their phytochemical concentration. The plant specimens were botanically identified by agricultural specialists at Gorgan University of Agricultural Sciences and Natural Resources. Following collection, the plant materials were meticulously cleaned and air-dried at ambient temperature in a shaded, well-ventilated space to prevent the degradation of volatile compounds.
Essential oil extraction
Essential oils were obtained from both male inflorescences and leaves of the plant using hydrodistillation. The extraction process was conducted with a Clevenger-type apparatus, adhering to the standards outlined in the European Pharmacopoeia. For each separate extraction, 100 grams of dried plant matter (Either male inflorescences or leaves) were combined with 1.5 liters of distilled water in a 2-liter round-bottom flask. The mixture was subjected to heating, and the resulting steam, laden with volatile oils, was subsequently condensed and collected. To ensure optimal recovery of the essential oils, the extraction process was sustained for a period of 4 hours. Given that the male inflorescences of this particular plant species contain aromatic compounds primarily during the plant’s fresh flowering stage, the extraction was executed immediately following the collection of fresh plant material to facilitate the capture of its volatile constituents. The essential oils were then isolated from the aqueous layer. Subsequently, they were dried using anhydrous sodium sulfate, filtered, and then placed in dark glass vials. To inhibit oxidative degradation, the samples were stored at 4°C. The essential oil yield was determined by calculating the ratio of the oils' weight to the dry weight of the plant material, expressed as a percentage (12).
Chemical analysis of essential oils
The chemical composition of the essential oils was determined through gas chromatography-mass spectrometry (GC-MS). The analysis was performed on a GC-MS system equipped with a fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Helium served as the carrier gas, maintaining a constant flow rate of 1 mL/min. The GC oven temperature was programmed to rise from 60°C to 240°C at a rate of 3°C/min, while the injector temperature was maintained at 250°C (13).
Bacterial strains and preparation
The antibacterial properties of the essential oils were evaluated against a panel of ten bacterial strains. These strains, which are known to be responsible for either foodborne illnesses or spoilage, included S. aureus (Persian Type Culture Collection [PTCC] 1917), E. coli (PTCC 1338), L. monocytogenes (PTCC 1783), P. aeruginosa (PTCC 1310), S. enteritidis (PTCC 1787), Shigella dysenteriae (S. dysenteriae) (PTCC 1188), Klebsiella pneumoniae (K. pneumoniae) (PTCC 1053), Alcaligenes faecalis (A. faecalis) (PTCC 1624), Serratia marcescens (S. marcescens) (PTCC 1621), and Streptococcus pyogenes (S. pyogenes) (PTCC 1762). All strains were procured from the PTCC. For each strain, a bacterial suspension was prepared by culturing it in brain-heart infusion (BHI) broth at 37°C for 18 hours to ensure it reached the exponential growth phase. The final bacterial concentration was standardized to approximately 10^6 colony-forming units (CFU)/mL by measuring the optical density at 600 nm with a spectrophotometer. This standardized inoculum was subsequently utilized in all downstream assays.
Antibacterial assays
Minimum inhibitory concentration and minimum bactericidal concentration
The MIC and MBC of the essential oils were established via the broth microdilution method. Serial twofold dilutions of the oils were prepared in 96-well microtiter plates, each containing 100 µL of BHI broth. After inoculating each well with 10 µL of a bacterial suspension adjusted to a concentration of 10^6 CFU/mL, the plates were incubated for 24 hours at 37°C. The MIC was determined as the lowest concentration of essential oil that totally prevented the visible growth of bacteria. To establish the MBC, samples from the wells with no visible growth were subcultured on nutrient agar. After 24 hours of incubation, the MBC was identified as the lowest concentration of essential oil that resulted in a 99.9% reduction in bacterial viability. All experiments were conducted in triplicate, and the mean values were reported (14).
Agar disk diffusion assay
The antibacterial efficacy of the essential oils was evaluated using the Clinical and Laboratory Standards Institute (CLSI) agar disk diffusion method. Overnight bacterial cultures were diluted in sterile saline to match a 0.5 McFarland standard, which corresponds to a density of approximately 10^6 CFU/mL. The bacterial suspension was uniformly distributed over Mueller-Hinton agar plates using a sterile cotton swab. Sterile, 6 mm paper disks, impregnated with 10 µL of essential oil (Diluted in dimethyl sulfoxide [DMSO]), were then placed on the inoculated surface. Following a 24-hour incubation at 37°C, the resulting zones of inhibition were measured with a digital caliper. For this study, gentamicin (10 µg/disc) and chloramphenicol (30 µg/disc) were utilized as positive controls, while disks containing only DMSO functioned as the negative control. All experiments were conducted in triplicate, and the final results are presented as the mean ± standard deviation (15).
Agar well diffusion assay
The agar well diffusion method was used to assess the antibacterial properties of the essential oils. First, holes measuring 6 mm in diameter were created in Mueller-Hinton agar plates that had already been seeded with bacterial suspensions. Next, 50 µL of various concentrations of essential oils (Diluted in DMSO) were added to each well. After incubating the plates at 37°C for 24 hours, the inhibition zones surrounding the wells were measured to determine the antibacterial effect. Positive and negative controls were incorporated into the experiment, similar to the disk diffusion assay. This technique enabled the evaluation of the antibacterial activity of different essential oil concentrations (16).
Statistical analysis
The antibacterial assay data were analyzed using SPSS software to evaluate the essential oils' efficacy against various bacterial strains. Prior to analysis, the Kolmogorov-Smirnov test was employed to confirm data normality, and Levene's test was used to verify homogeneity of variances. To determine statistically significant differences among the groups, a one-way analysis of variance (ANOVA) was performed, followed by Tukey's post hoc test. The level of significance was established at p < 0.05. All results were presented as the mean ± standard deviation from three independent experiments.
Results
This study’s findings reveal the chemical composition of S. aegyptiaca essential oils derived from the leaves and male inflorescences. The research also details the oils' antibacterial efficacy against several foodborne pathogens. These results are reported through an analysis of the chemical profile, MIC and MBC values, and the zones of inhibition recorded in both agar disk and well diffusion assays.
Chemical composition of salix aegyptiaca essential oils
GC-MS analysis of the essential oils extracted from both the leaves and male inflorescences revealed a variety of bioactive compounds. According to Table 1, the main compounds in the leaf oil were 1,4-dimethoxybenzene (34.78%), citronellol (13.53%), and eugenol (5.29%). In contrast, the male inflorescence oil was predominantly composed of 1,4-dimethoxybenzene (28.46%), followed by citronellol (10.75%) and carvone (5.12%). The antimicrobial properties of these compounds likely account for the efficacy of the oils against the bacterial strains examined in this study. The observed variations in the chemical composition of the oils derived from the leaves versus the male inflorescences indicate that the biological activity of the essential oils may be dependent on their specific source within the plant (17,18).
Minimum inhibitory concentration and minimum bactericidal concentration results
Table 2 summarizes the MIC and MBC values for each bacterial strain. These values indicate the specific oil concentrations required to inhibit the growth of, and to kill each strain, respectively.
Leaf essential oil
The MIC values for the leaf oil demonstrated a range from 1250 µg/mL against S. aureus to 5000 µg/mL for both S. enteritidis and S. dysenteriae. Similarly, the MBC values followed a comparable pattern, with the lowest concentration (2500 µg/mL) required to inhibit S. aureus, while S. enteritidis and S. dysenteriae needed a higher concentration of 5000 µg/mL for bactericidal effects. These findings suggest that S. aureus exhibits the highest susceptibility to the leaf oil, whereas S. enteritidis and S. dysenteriae are considerably more resistant.
Male inflorescence essential oil
The MIC and MBC values for the male inflorescence oil were consistently higher than those for the leaf oil, suggesting a diminished overall antimicrobial efficacy. The oil demonstrated its most potent inhibitory effect against S. marcescens and S. aureus, for which the lowest MIC of 2500 µg/mL was recorded. In contrast, the highest MIC of 5000 µg/mL was required to inhibit the growth of E. coli, S. enteritidis, and S. dysenteriae. For bactericidal activity, the MBC for S. marcescens and S. aureus was also at its lowest, at 2500 µg/mL. However, a significantly higher concentration of 10,000 µg/mL was necessary to achieve a bactericidal effect against S. enteritidis.
Agar disk diffusion assay
Inhibition zones, which varied by bacterial strain and essential oil type, were observed in the agar disk diffusion assay (Table 3). The leaf oil consistently produced larger inhibition zones compared to the male inflorescence oil.
Leaf oil
The extract exhibited its most potent antibacterial activity against S. aureus, as evidenced by the largest mean inhibition zone diameter of 9.38 ± 0.15 mm. Conversely, E. coli displayed the least susceptibility to the extract, with the smallest observed inhibition zone of 7.59 ± 0.20 mm. The other tested strains, including P. aeruginosa and S. marcescens, demonstrated moderate sensitivity, producing inhibition zones of 9.28 ± 0.15 mm and 9.12 ± 0.15 mm, respectively.
|
Table 1. Chemical composition of Salix aegyptiaca essential oils (Gas chromatography-mass spectrometry analysis)
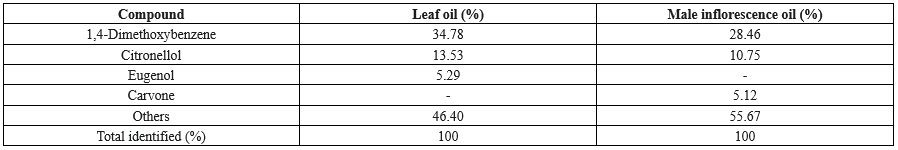 Table 2. Minimum inhibitory concentration and minimum bactericidal concentration of Salix aegyptiaca essential oils against bacterial strains 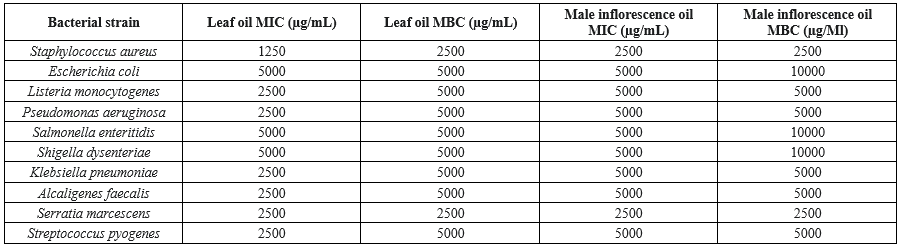 Table 3. Inhibition zones of Salix aegyptiaca essential oils (Agar disk diffusion) 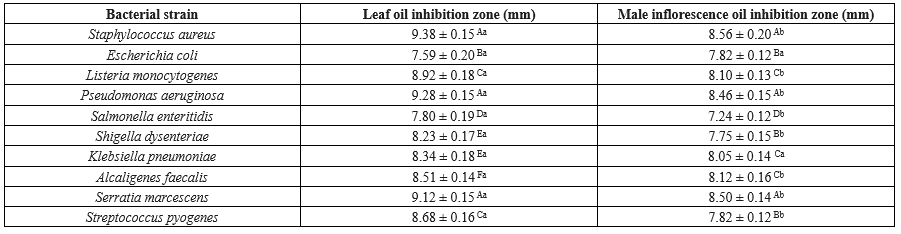 Different capital letters in each column indicate a statistically significant difference (P < 0.05) Different small letters in each row indicate a statistically significant difference (P < 0.05) |
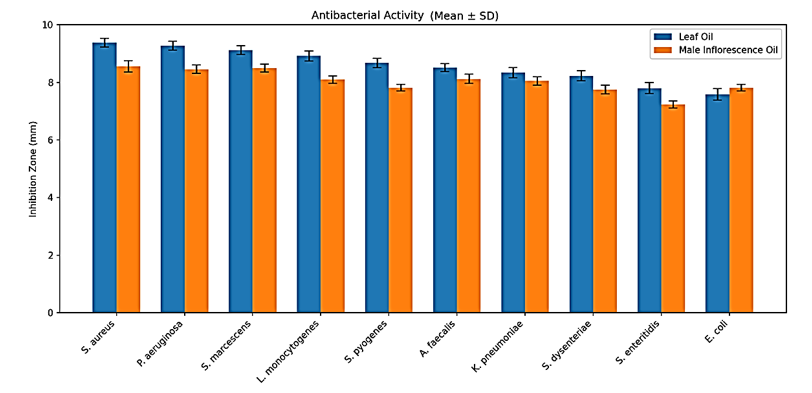 Figure 1. Comparative antimicrobial activity of Salix aegyptiaca leaf and male inflorescence essential oils against various bacterial strains, as measured by inhibition zone diameters (mm) using the agar well diffusion method |
Male inflorescence oil
The inhibitory effects of the male inflorescence oil were less pronounced than those of the leaf oil, with generally smaller inhibition zones. S. aureus demonstrated the highest susceptibility to the male inflorescence oil, yielding the largest inhibition zone at 8.56 ± 0.20 mm. Conversely, S. pyogenes exhibited the greatest resistance, showing the smallest inhibition zone (7.82 ± 0.12 mm). These findings corroborate that S. aureus is highly susceptible to both oils, though the leaf oil appears to be marginally more effective.
Agar well diffusion assay
The results from the agar well diffusion assay corroborated the antibacterial activity noted in the disk diffusion test and additionally revealed varying degrees of susceptibility among the bacterial strains (Figure 1).
Leaf oil
Based on the results of the well diffusion assay presented in Figure 1, S. aureus was the most susceptible bacterium, as indicated by its large inhibition zone of 9.38 ± 0.15 mm. In contrast, E. coli demonstrated the highest resistance, with the smallest inhibition zone measuring 7.59 ± 0.20 mm. P. aeruginosa and S. marcescens showed moderate susceptibility with inhibition zones of 9.28 ± 0.15 mm and 9.12 ± 0.15 mm, respectively, demonstrating that the antibacterial effectiveness of the tested agent varies depending on the specific bacterial species.
Male inflorescence oil
In the agar well diffusion assay, the male inflorescence oil showed similar trends, exhibiting the most significant inhibition against S. aureus (8.56 ± 0.20 mm) and the least against S. dysenteriae (7.24 ± 0.12 mm). The consistent results across both diffusion methods confirm that S. aureus is the most susceptible bacterium. However, the male inflorescence oil's efficacy was slightly less than that of the leaf oil. Statistical analysis revealed a significant difference (p < 0.05) in the antibacterial efficacy of essential oils derived from the leaves and male inflorescences against specific bacterial strains. The variation in inhibition zones, MIC, and MBC values was statistically significant across the strains. S. aureus consistently showed greater susceptibility, while E. coli and S. enteritidis demonstrated lower sensitivity to both essential oils. These results highlight the potential of S. aegyptiaca essential oils, especially the leaf oil, as potent antimicrobial agents.
Discussion
This research offers a comparative investigation into the chemical composition and antibacterial efficacy of essential oils sourced from both the leaves and male inflorescences of S. aegyptiaca. The study tested these oils against various bacterial strains known to cause foodborne illnesses and food spoilage. The results demonstrate that the essential oils, especially those derived from the leaves, exhibit considerable antibacterial action against a range of Gram-positive and Gram-negative bacteria. However, the effectiveness of the oils differs across bacterial species (19). While a lot of research has been conducted on the antibacterial properties of plant extracts, this study is unique because it specifically compares the potential of different parts of S. aegyptiaca, a plant known for its medicinal uses. It tests these extracts against a variety of important foodborne pathogens. The findings show that the chemical composition and antibacterial activity of the leaf and male inflorescence oils differ, helping us understand how to use this plant most effectively.
The GC-MS analysis demonstrated that 1,4-dimethoxybenzene, citronellol, and eugenol were the primary constituents of the leaf oil. In contrast, the male inflorescence oil w:as char:acterized by a high concentration of 1,4-dimethoxybenzene, citronellol, and carvone. These compounds are recognized for their antimicrobial activity, which is thought to be a result of their chemical structures and their ability to interact with bacterial cell membranes. Eugenol, a phenolic compound, and citronellol, a monoterpenoid, have demonstrated the ability to kill bacteria by disrupting cell walls, increasing membrane permeability, and interfering with essential intracellular processes (20,21). Additionally, 1,4-dimethoxybenzene has been shown to possess antimicrobial properties, although its effectiveness is strain- and concentration-dependent. The elevated percentage of this compound in the leaf oil, alongside the presence of eugenol (Which was absent in the male inflorescence oil), may explain its superior antibacterial activity. This finding is consistent with research indicating that the efficacy of essential oils is often directly tied to the concentration and synergistic effects of their active components (22). While the distinct chemical profile of the male inflorescence oil, particularly the presence of carvone, also contributes to its antimicrobial properties, its effect was less pronounced than that of the leaf oil in this study.
The findings revealed that S. aureus (A Gram-positive bacterium) was the most vulnerable to the oils from both the leaves and the male inflorescences. In contrast, the Gram-negative bacteria E. coli and S. enteritidis showed greater resistance, as evidenced by their elevated MIC and MBC values. This differential susceptibility is a commonly observed phenomenon and can be attributed to the inherent structural differences between Gram-positive and Gram-negative bacteria (23). The presence of an outer lipopolysaccharide layer present in Gram-negative bacteria acts as a protective barrier, impeding the entry of hydrophobic molecules, including compounds found in essential oils (24). In contrast, Gram-positive bacteria lack this outer membrane, which allows for easier access of essential oils to their cell wall and plasma membrane. This structural difference explains why essential oils are generally more effective against Gram-positive bacteria, a finding consistent with numerous other studies (25).
The essential oils derived from S. aegyptiaca likely combat bacteria through several pathways. Research suggests that phenolic compounds, for instance, eugenol, disrupt the cell membrane, denature proteins, and inhibit enzyme activity (26). Furthermore, terpenoids, such as citronellol and carvone, are known to damage the cell walls and membranes of bacteria, thereby increasing their permeability and causing the leakage of essential cellular materials (27). Due to their multi-target mechanisms of action, essential oils hold significant promise as antimicrobial agents. This is because the simultaneous application of multiple stressors makes it more difficult for bacteria to develop resistance (28). The diverse chemical composition observed in essential oils derived from leaves and male inflorescences suggests that their antimicrobial efficacy likely stems from synergistic interactions among their various constituents. This synergy may amplify the overall activity of the oil, surpassing the simple additive effects of its individual components (29).
The disk and well diffusion assays demonstrated that essential oils extracted from S. aegyptiaca leaves created substantial inhibition zones against S. aureus. While these zones were generally smaller than those produced by the positive control, gentamicin, the natural origin of essential oils, their lower potential for inducing antibiotic resistance, and their higher consumer acceptance in the organic food market provide them with distinct advantages over synthetic antibiotics (30,31). Antibiotics typically focus on specific cellular pathways, which can culminate in resistance over time. In contrast, essential oils work through a wider range of mechanisms, affecting multiple targets within bacterial cells. This broader action may lower the chances of resistance emerging, positioning essential oils as a promising alternative for fighting antibiotic-resistant bacterial strains (32,33).
The rationale for this study is its prospective contribution to the food sector through the investigation of natural alternatives for food preservation. This field is of increasing interest given consumer demand for and concerns about synthetic additives. Although this research has established the promising antibacterial efficacy of S. aegyptiaca essential oils, certain limitations warrant consideration. The study’s in vitro design may not accurately reflect the oil’s efficacy within complex food systems, as interactions with other components could alter its performance. Therefore, future research should explore the oils’ antimicrobial properties in real food matrices to better evaluate their potential as practical preservatives. Additionally, while the chemical composition of the oils was analyzed, the potential synergistic effects of their individual components were not specifically investigated. Fractionation studies and combination assays of these individual compounds would offer a more detailed understanding of how specific bioactive components contribute to the overall effect. Crucially, toxicity studies are indispensable to confirm that the application of these essential oils at antimicrobial concentrations does not endanger human health. While the discovery that different plant parts possess distinct oil compositions and bioactivities may not be groundbreaking in a general context, the specific data comparing the efficacy of S. aegyptiaca male inflorescence and leaf oils against various foodborne pathogens contributes significant, novel information to the existing literature on plant-derived antimicrobials.
Conclusion
The research findings indicate that essential oils obtained from the leaves and male inflorescences of S. aegyptiaca show significant antibacterial properties against several foodborne pathogens. The leaf essential oil, characterized by a high content of 1,4-dimethoxybenzene, citronellol, and eugenol, was more effective than the oil extracted from the male inflorescence. S. aureus was identified as the most susceptible bacterium to the treatment, whereas Gram-negative bacteria such as E. coli demonstrated higher levels of resistance. The essential oils of S. aegyptiaca, especially those derived from the leaves, show promise as natural antimicrobial agents. These oils could be used in food preservation, for instance by integrating them into packaging or adding them directly to food products to prolong shelf life and improve safety. Further research should prioritize in-situ investigations within food matrices to assess synergistic effects and conduct exhaustive toxicological evaluations. These steps are essential to enable the safe and effective use of these compounds as an alternative to synthetic preservatives in the food industry.
Acknowledgement
This research was funded by Golestan University of Medical Sciences, Gorgan, Iran (Project No. 113373).
Funding sources
We confirm that no funding was provided for this work.
Ethical statement
In this study, no clinical trials involving human or animal subjects were conducted. Consequently, an ethics statement concerning patient consent or clinical trial registration is not relevant.
Conflicts of interest
No conflict of interest.
Author contributions
MR, FH, and MG: Writing - original draft, methodology, investigation, formal analysis, and conceptualization; MAM: Writing - original draft and investigation; NM: Writing - review and editing and writing - original draft.
Data availability statement
Not applicable.
The inhibitory effects of the male inflorescence oil were less pronounced than those of the leaf oil, with generally smaller inhibition zones. S. aureus demonstrated the highest susceptibility to the male inflorescence oil, yielding the largest inhibition zone at 8.56 ± 0.20 mm. Conversely, S. pyogenes exhibited the greatest resistance, showing the smallest inhibition zone (7.82 ± 0.12 mm). These findings corroborate that S. aureus is highly susceptible to both oils, though the leaf oil appears to be marginally more effective.
Agar well diffusion assay
The results from the agar well diffusion assay corroborated the antibacterial activity noted in the disk diffusion test and additionally revealed varying degrees of susceptibility among the bacterial strains (Figure 1).
Leaf oil
Based on the results of the well diffusion assay presented in Figure 1, S. aureus was the most susceptible bacterium, as indicated by its large inhibition zone of 9.38 ± 0.15 mm. In contrast, E. coli demonstrated the highest resistance, with the smallest inhibition zone measuring 7.59 ± 0.20 mm. P. aeruginosa and S. marcescens showed moderate susceptibility with inhibition zones of 9.28 ± 0.15 mm and 9.12 ± 0.15 mm, respectively, demonstrating that the antibacterial effectiveness of the tested agent varies depending on the specific bacterial species.
Male inflorescence oil
In the agar well diffusion assay, the male inflorescence oil showed similar trends, exhibiting the most significant inhibition against S. aureus (8.56 ± 0.20 mm) and the least against S. dysenteriae (7.24 ± 0.12 mm). The consistent results across both diffusion methods confirm that S. aureus is the most susceptible bacterium. However, the male inflorescence oil's efficacy was slightly less than that of the leaf oil. Statistical analysis revealed a significant difference (p < 0.05) in the antibacterial efficacy of essential oils derived from the leaves and male inflorescences against specific bacterial strains. The variation in inhibition zones, MIC, and MBC values was statistically significant across the strains. S. aureus consistently showed greater susceptibility, while E. coli and S. enteritidis demonstrated lower sensitivity to both essential oils. These results highlight the potential of S. aegyptiaca essential oils, especially the leaf oil, as potent antimicrobial agents.
Discussion
This research offers a comparative investigation into the chemical composition and antibacterial efficacy of essential oils sourced from both the leaves and male inflorescences of S. aegyptiaca. The study tested these oils against various bacterial strains known to cause foodborne illnesses and food spoilage. The results demonstrate that the essential oils, especially those derived from the leaves, exhibit considerable antibacterial action against a range of Gram-positive and Gram-negative bacteria. However, the effectiveness of the oils differs across bacterial species (19). While a lot of research has been conducted on the antibacterial properties of plant extracts, this study is unique because it specifically compares the potential of different parts of S. aegyptiaca, a plant known for its medicinal uses. It tests these extracts against a variety of important foodborne pathogens. The findings show that the chemical composition and antibacterial activity of the leaf and male inflorescence oils differ, helping us understand how to use this plant most effectively.
The GC-MS analysis demonstrated that 1,4-dimethoxybenzene, citronellol, and eugenol were the primary constituents of the leaf oil. In contrast, the male inflorescence oil w:as char:acterized by a high concentration of 1,4-dimethoxybenzene, citronellol, and carvone. These compounds are recognized for their antimicrobial activity, which is thought to be a result of their chemical structures and their ability to interact with bacterial cell membranes. Eugenol, a phenolic compound, and citronellol, a monoterpenoid, have demonstrated the ability to kill bacteria by disrupting cell walls, increasing membrane permeability, and interfering with essential intracellular processes (20,21). Additionally, 1,4-dimethoxybenzene has been shown to possess antimicrobial properties, although its effectiveness is strain- and concentration-dependent. The elevated percentage of this compound in the leaf oil, alongside the presence of eugenol (Which was absent in the male inflorescence oil), may explain its superior antibacterial activity. This finding is consistent with research indicating that the efficacy of essential oils is often directly tied to the concentration and synergistic effects of their active components (22). While the distinct chemical profile of the male inflorescence oil, particularly the presence of carvone, also contributes to its antimicrobial properties, its effect was less pronounced than that of the leaf oil in this study.
The findings revealed that S. aureus (A Gram-positive bacterium) was the most vulnerable to the oils from both the leaves and the male inflorescences. In contrast, the Gram-negative bacteria E. coli and S. enteritidis showed greater resistance, as evidenced by their elevated MIC and MBC values. This differential susceptibility is a commonly observed phenomenon and can be attributed to the inherent structural differences between Gram-positive and Gram-negative bacteria (23). The presence of an outer lipopolysaccharide layer present in Gram-negative bacteria acts as a protective barrier, impeding the entry of hydrophobic molecules, including compounds found in essential oils (24). In contrast, Gram-positive bacteria lack this outer membrane, which allows for easier access of essential oils to their cell wall and plasma membrane. This structural difference explains why essential oils are generally more effective against Gram-positive bacteria, a finding consistent with numerous other studies (25).
The essential oils derived from S. aegyptiaca likely combat bacteria through several pathways. Research suggests that phenolic compounds, for instance, eugenol, disrupt the cell membrane, denature proteins, and inhibit enzyme activity (26). Furthermore, terpenoids, such as citronellol and carvone, are known to damage the cell walls and membranes of bacteria, thereby increasing their permeability and causing the leakage of essential cellular materials (27). Due to their multi-target mechanisms of action, essential oils hold significant promise as antimicrobial agents. This is because the simultaneous application of multiple stressors makes it more difficult for bacteria to develop resistance (28). The diverse chemical composition observed in essential oils derived from leaves and male inflorescences suggests that their antimicrobial efficacy likely stems from synergistic interactions among their various constituents. This synergy may amplify the overall activity of the oil, surpassing the simple additive effects of its individual components (29).
The disk and well diffusion assays demonstrated that essential oils extracted from S. aegyptiaca leaves created substantial inhibition zones against S. aureus. While these zones were generally smaller than those produced by the positive control, gentamicin, the natural origin of essential oils, their lower potential for inducing antibiotic resistance, and their higher consumer acceptance in the organic food market provide them with distinct advantages over synthetic antibiotics (30,31). Antibiotics typically focus on specific cellular pathways, which can culminate in resistance over time. In contrast, essential oils work through a wider range of mechanisms, affecting multiple targets within bacterial cells. This broader action may lower the chances of resistance emerging, positioning essential oils as a promising alternative for fighting antibiotic-resistant bacterial strains (32,33).
The rationale for this study is its prospective contribution to the food sector through the investigation of natural alternatives for food preservation. This field is of increasing interest given consumer demand for and concerns about synthetic additives. Although this research has established the promising antibacterial efficacy of S. aegyptiaca essential oils, certain limitations warrant consideration. The study’s in vitro design may not accurately reflect the oil’s efficacy within complex food systems, as interactions with other components could alter its performance. Therefore, future research should explore the oils’ antimicrobial properties in real food matrices to better evaluate their potential as practical preservatives. Additionally, while the chemical composition of the oils was analyzed, the potential synergistic effects of their individual components were not specifically investigated. Fractionation studies and combination assays of these individual compounds would offer a more detailed understanding of how specific bioactive components contribute to the overall effect. Crucially, toxicity studies are indispensable to confirm that the application of these essential oils at antimicrobial concentrations does not endanger human health. While the discovery that different plant parts possess distinct oil compositions and bioactivities may not be groundbreaking in a general context, the specific data comparing the efficacy of S. aegyptiaca male inflorescence and leaf oils against various foodborne pathogens contributes significant, novel information to the existing literature on plant-derived antimicrobials.
Conclusion
The research findings indicate that essential oils obtained from the leaves and male inflorescences of S. aegyptiaca show significant antibacterial properties against several foodborne pathogens. The leaf essential oil, characterized by a high content of 1,4-dimethoxybenzene, citronellol, and eugenol, was more effective than the oil extracted from the male inflorescence. S. aureus was identified as the most susceptible bacterium to the treatment, whereas Gram-negative bacteria such as E. coli demonstrated higher levels of resistance. The essential oils of S. aegyptiaca, especially those derived from the leaves, show promise as natural antimicrobial agents. These oils could be used in food preservation, for instance by integrating them into packaging or adding them directly to food products to prolong shelf life and improve safety. Further research should prioritize in-situ investigations within food matrices to assess synergistic effects and conduct exhaustive toxicological evaluations. These steps are essential to enable the safe and effective use of these compounds as an alternative to synthetic preservatives in the food industry.
Acknowledgement
This research was funded by Golestan University of Medical Sciences, Gorgan, Iran (Project No. 113373).
Funding sources
We confirm that no funding was provided for this work.
Ethical statement
In this study, no clinical trials involving human or animal subjects were conducted. Consequently, an ethics statement concerning patient consent or clinical trial registration is not relevant.
Conflicts of interest
No conflict of interest.
Author contributions
MR, FH, and MG: Writing - original draft, methodology, investigation, formal analysis, and conceptualization; MAM: Writing - original draft and investigation; NM: Writing - review and editing and writing - original draft.
Data availability statement
Not applicable.
Research Article: Research Article |
Subject:
Microbiology
Received: 2025/02/26 | Accepted: 2025/06/8 | Published: 2025/09/17 | ePublished: 2025/09/17
Received: 2025/02/26 | Accepted: 2025/06/8 | Published: 2025/09/17 | ePublished: 2025/09/17
References
1. Coque TM, Cantón R, Pérez-Cobas AE, Fernández-de-Bobadilla MD, Baquero F. Antimicrobial resistance in the global health network: known unknowns and challenges for efficient responses in the 21st century. Microorganisms. 2023;11(4):1050. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Angelini P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics. 2024;13(8):746. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Asgarpanah J. Phytopharmacology and medicinal properties of Salix aegyptiaca L. Afr J Biotechnol. 2012;11(28):7145-50. [View at Publisher] [Google Scholar]
4. Tawfeek N, Mahmoud MF, Hamdan DI, Sobeh M, Farrag N, Wink M, et al. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front Pharmacol. 2021;12:593856. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Chothani DL, Vaghasiya HU. A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn Rev. 2011;5(9):55-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Böhme K, Barros-Velázquez J, Calo-Mata P, Aubourg SP. Antibacterial, antiviral and antifungal activity of essential oils: Mechanisms and applications. Antimicrob Comb. 2013;51-81. [View at Publisher] [DOI] [Google Scholar]
7. Popova TP, Kaleva MD. Antimicrobial effect in vitro of aqueous extracts of leaves and branches of willow (Salix babylonica L.). Int J Curr Microbiol Appl Sci. 2015;4(10):146-52. [View at Publisher] [Google Scholar]
8. Javed B, Farooq F, Ibrahim M, Abbas H, Jawwad H, Zehra S, et al. Antibacterial and antifungal activity of methanolic extracts of Salix alba L. against various disease causing pathogens. Braz J Biol. 2021;83:e243332. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Ghalem BR. Essential oils as antimicrobial agents against some important plant pathogenic bacteria and fungi. Plant-Microbe Interaction: An Approach to Sustainable Agriculture. 2017:271-96. [View at Publisher] [DOI] [Google Scholar]
10. Rasheed HA, Rehman A, Karim A, Al-Asmari F, Cui H, Lin L. A comprehensive insight into plant-derived extracts/bioactives: Exploring their antimicrobial mechanisms and potential for high-performance food applications. Food Bioscience. 2024:104035. [View at Publisher] [DOI] [Google Scholar]
11. Hulankova R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro-A Review. Plants. 2024;13(19):2784. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Shamspur T, Sheikhshoaie I, Afzali D, Mostafavi A, Mirtadzadini S. Chemical Compositions of Salix aegyptiaca. L. Obtained by Simultaneous Hydrodistilation and Extraction. Journal of Essential Oil Bearing Plants. 2011;14(5):543-8. [View at Publisher] [DOI] [Google Scholar]
13. Karimi I, Hayatgheybi H, Kamalak A, Pooyanmehr M, Marandi Y. Chemical composition and effect of an essential oil of Salix aegyptiaca L., Salicaceae,(musk willow) in hypercholesterolemic rabbit model. Rev Bras Farmacogn. 2011;21(3):407-14. [View at Publisher] [DOI] [Google Scholar]
14. Galgano M, Capozza P, Pellegrini F, Cordisco M, Sposato A, Sblano S, et al. Antimicrobial activity of essential oils evaluated in vitro against Escherichia coli and Staphylococcus aureus. Antibiotics. 2022;11(7):979. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Humphries RM, Kircher S, Ferrell A, Krause KM, Malherbe R, Hsiung A, et al. The continued value of disk diffusion for assessing antimicrobial susceptibility in clinical laboratories: report from the clinical and laboratory standards institute methods development and standardization working group. J Clin Microbiol. 2018;56(8):00437-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58(3):e01864-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Gano M, Janus E, Cybulska K. Effect of terpene alcohol fragment and type of anion in the pyrrolidinium salts on the physicochemical properties and antibacterial activity. Journal of Molecular Liquids. 2024;407(1):125254. [View at Publisher] [DOI] [Google Scholar]
18. Wróblewska A, Fajdek-Bieda A, Markowska-Szczupak A, Radkowska M. Preliminary microbiological tests of s-carvone and geraniol and selected derivatives of these compounds that may be formed in the processes of isomerization and oxidation. Molecules. 2022;27(20):7012. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Abdalrahman MR, Sofi SA. The Bark Extraction of Salix Aegyptiaca L Antibacterial Activity. The journal of contemporary issues in business and government. 2021;27(3):169-71. [View at Publisher] [DOI] [Google Scholar]
20. Milovanovic S, Markovic D, Jankovic-Castvan I, Lukic I. Cornstarch aerogels with thymol, citronellol, carvacrol, and eugenol prepared by supercritical CO2-assisted techniques for potential biomedical applications. Carbohydr Polym. 2024;331:121874. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Olszewska MA, Gędas A, Simões M. The effects of eugenol, trans-cinnamaldehyde, citronellol, and terpineol on Escherichia coli biofilm control as assessed by culture-dependent and-independent methods. Molecules. 2020;25(11):2641. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Casertano M, Aiello A, Menna M, Imperatore C. The Effects of Ceric Ammonium Nitrate in the Oxidation of 2-Benzyl-1, 4-dimethoxybenzene Derivatives. Molbank. 2024;2024(3):M1882. [View at Publisher] [DOI] [Google Scholar]
23. Andrade-Ochoa S, Chacón-Vargas KF, Sánchez-Torres LE, Rivera-Chavira BE, Nogueda-Torres B, Nevárez-Moorillón GV. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes. 2021;11(6):405. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Liu M, Cheng J-H, Zhao H, Yu C, Wu J. Targeting the outer membrane of gram-negative foodborne pathogens for food safety: compositions, functions, and disruption strategies. Crit Rev Food Sci Nutr. 2024:1-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Nourbakhsh F, Lotfalizadeh M, Badpeyma M, Shakeri A, Soheili V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother Res. 2022;36(1):33-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Niu D, Wang Q-Y, Ren E-F, Zeng X-A, Wang L-H, He T-F, et al. Multi-target antibacterial mechanism of eugenol and its combined inactivation with pulsed electric fields in a hurdle strategy on Escherichia coli. Food Control. 2019;106:106742. [View at Publisher] [DOI] [Google Scholar]
27. Lopez-Romero JC, González-Ríos H, Borges A, Simões M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015:2015:795435. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Bunse M, Daniels R, Gründemann C, Heilmann J, Kammerer DR, Keusgen M, et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front Pharmacol. 2022;13:956541. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Ghavam M, Manca ML, Manconi M, Bacchetta G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci Rep. 2020;10(1):15647. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Iseppi R, Mariani M, Condò C, Sabia C, Messi P. Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics. 2021;10(4):417. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Zhang L, Gao F, Ge J, Li H, Xia F, Bai H, et al. Potential of aromatic plant-derived essential oils for the control of foodborne bacteria and antibiotic resistance in animal production: a review. Antibiotics. 2022;11(11):1673. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2021;10(10):1310. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Angane M, Swift S, Huang K, Butts CA, Quek SY. Essential oils and their major components: an updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods. 2022;11(3):464. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com