Volume 19, Issue 4 (Jul-Aug 2025)
mljgoums 2025, 19(4): 5-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gowda R Y, Kumar S A, Srevatsa K. Prevalence and trends of hepatitis B infection among blood donors. mljgoums 2025; 19 (4) :5-8
URL: http://mlj.goums.ac.ir/article-1-1765-en.html
URL: http://mlj.goums.ac.ir/article-1-1765-en.html
1- Department of Pathology, Nandi Medical College, Aroor, Chikkaballapur, Karnataka, India , yashica317@gmail.com
2- Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India
3- Department of Pathology, Nandi Medical College, Aroor, Chikkaballapur, Karnataka, India
2- Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India
3- Department of Pathology, Nandi Medical College, Aroor, Chikkaballapur, Karnataka, India
Full-Text [PDF 426 kb]
(695 Downloads)
| Abstract (HTML) (2601 Views)
Discussion
Viral hepatitis is one of the serious complications of blood transfusion, most commonly caused by hepatitis B and hepatitis C viruses. Humans are the only reservoirs of HBV. It is a DNA virus, first discovered by Blumberg et al. in 1965 in the serum of an Australian aborigine (8).
To reduce the risk of transfusion-transmissible diseases, the World Health Organization recommends mandatory blood screening before transfusion (7). The risk of TTIs has declined dramatically in developed countries over the last two decades. However, blood screening in developing countries like India largely depends on ELISA, which targets only HBsAg and cannot detect occult hepatitis, continuing the risk of transfusion-transmitted hepatitis B infection. In recent years, the National Viral Hepatitis Control Programme has been initiated under the National Health Mission of India, for the prevention and control of viral hepatitis, with a view to providing free screening, diagnosis, counselling, and appropriate treatment, especially to people belonging to high-risk groups. Recent advances in diagnostics have now made it possible to diagnose hepatitis infection through point-of-care diagnostic kits. Several new technologies and platforms are also now available for conducting confirmatory tests through viral load testing (9).
The overall seroprevalence of HBsAg in our study was 0.66%. This was comparable to studies done by Singh et al. (0.62%) (10) and Dhruva G. A. et al. (0.97%) (11). However, a higher prevalence was noted in studies done in Rajasthan (3.44%) (12) and Delhi (2.22%) (13) (Figure 2), while the prevalence in other geographic areas is tabulated in Table 1. All the studies done across India show a higher prevalence of HBsAg among replacement donors, as the replacement method of blood collection was widely practiced until recent years. The variation in prevalence among different studies is probably due to differences in social behavior, lifestyle, socioeconomic status, immunization status, and the level of awareness regarding the infection.
Conclusion
Our study shows a declining trend and prevalence of HBsAg seropositivity among blood donors, which is an encouraging sign, reflecting the effectiveness of strict donor selection criteria, the conduction of blood donation camps, and the use of sensitive screening tests. Although seropositivity can be further reduced by implementing nucleic acid amplification test and polymerase chain reaction, these diagnostic tests are financially and technically beyond reach in developing countries like India. To conclude, promoting and encouraging voluntary blood donation is a simple and effective way to reduce the prevalence of all TTIs.
Acknowledgement
We extend our gratitude to the institution and the blood center staff for their invaluable contribution to the study.
Funding sources
None.
Ethical statement
Permission was obtained from the Institutional Ethics Committee.
Conflicts of interest
None.
Author contributions
Yashica Gowda and Suja Ajoy Kumar conceived the presented idea. Yashica Gowda and Karthik Srevatsa verified the analytical methods. Suja Ajoy Kumar encouraged the investigation and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Full-Text: (434 Views)
Introduction
The importance of blood transfusion can be described by the phrase, "It’s not just blood, its liquid life." It is rightly said, as there is no veritable substitution for blood, and it plays an important role in the supportive care of patients. However, it carries its own risks if blood safety is not ensured. Transmission of infections is one such risk factor (1). Viral infectious agents such as hepatitis B virus (HBV), hepatitis C virus, and human immunodeficiency virus are of the greatest concern. Among these, hepatitis B infection is highly contagious and transmitted through exposure to infected blood and body fluids, sexual intercourse, and maternofetal transmission (2). In spite of the availability of an effective vaccine against HBV, it remains a major public health concern due to its high infectivity, chronicity, and mortality.
In July 2022, the Centers for Disease Control reported that approximately 296 million people were chronically infected with hepatitis B, including 6 million children under the age of three years, contributing to approximately 820,000 deaths every year. Twenty-five percent of chronic hepatitis B infections progress to liver cancer (3). The prevalence of these viral infections among blood donors shows a wide range of variation across different geographic areas (2). According to the World Health Organization, the prevalence of HBV among blood donors in different parts of the world varies from 0.008% to 6.08% (2,4).
Donor screening strategies and the prevalence of risk factors in the society account for the variation in prevalence rates over time. Currently, the incidence of HBV infection through blood transfusion is relatively low, as screening of blood for transfusion-transmitted infections (TTIs) is the highest priority. However, the residual risk of HBV transmission is associated with the preseroconversion window period and the presence of occult HBV infection, which is characterized by the absence of detectable hepatitis B surface antigen (HBsAg) (5,6). The global incidence of occult hepatitis among blood donors ranges from 0.006% to 17.2% (7).
The high demand for blood transfusion warrants continuous monitoring of the magnitude of transfusion-transmitted infections in blood donors (2). The screening of TTIs in blood centers not only reduces the risk of HBV transmission through transfusion but also provides insight into the prevalence of infection in the healthy population.
The present study was conducted to determine the trend of HBsAg seropositivity among the blood donor population during a five-year period and to demonstrate the difference in prevalence of HBsAg among voluntary and replacement donors. Replacement donors are one-time blood donors who donate blood only when a relative is in need of blood.
Methods
This study was conducted at a licensed blood center of a tertiary care hospital in Bengaluru, India. A retrospective review of blood donor data from January 2018 to December 2022 was extracted. The annual total blood donors were classified into voluntary and replacement donors. All donated blood was screened for the presence of HBsAg using a commercially available ELISA kit (Qualisa, Microwell Enzyme Immunoassay, Qualpro Diagnostics, India). All initially positive samples were retested, and the repeatedly reactive samples were labelled seropositive. According to the National Blood Transfusion Committee policy, positive blood units were discarded, and donors were recalled for counselling and treatment. The data were analyzed for trends in the prevalence of HBsAg. Ethical clearance was obtained from the Institutional Ethics Committee (Ref. no. IEC/A-32/23) before the start of the study, according to the principles of the Declaration of Helsinki.
Results
A total of 18,139 apparently healthy donors were screened during the study period. Among them, 11,517 were replacement donors and 6,622 were voluntary donors, yearly distribution as shown in Figure 1. The overall prevalence of HBsAg seropositivity was 0.66% (Figure 2). The distribution of HBsAg seropositivity among replacement and voluntary donors was 85 and 36, respectively, as depicted in Figure 3. The predominant age group of seropositive donors is shown in Figure 4, with a predominance in the age group of 20 to 40 years. The trends in the seroprevalence of HBsAg during the five-year period are shown in Figure 2, which depict a decline from 0.83% to 0.5%. The lowest seroprevalence was noted in 2022. The annual variation of HBsAg seroprevalence among replacement and voluntary donors is depicted in Figure 3.
The importance of blood transfusion can be described by the phrase, "It’s not just blood, its liquid life." It is rightly said, as there is no veritable substitution for blood, and it plays an important role in the supportive care of patients. However, it carries its own risks if blood safety is not ensured. Transmission of infections is one such risk factor (1). Viral infectious agents such as hepatitis B virus (HBV), hepatitis C virus, and human immunodeficiency virus are of the greatest concern. Among these, hepatitis B infection is highly contagious and transmitted through exposure to infected blood and body fluids, sexual intercourse, and maternofetal transmission (2). In spite of the availability of an effective vaccine against HBV, it remains a major public health concern due to its high infectivity, chronicity, and mortality.
In July 2022, the Centers for Disease Control reported that approximately 296 million people were chronically infected with hepatitis B, including 6 million children under the age of three years, contributing to approximately 820,000 deaths every year. Twenty-five percent of chronic hepatitis B infections progress to liver cancer (3). The prevalence of these viral infections among blood donors shows a wide range of variation across different geographic areas (2). According to the World Health Organization, the prevalence of HBV among blood donors in different parts of the world varies from 0.008% to 6.08% (2,4).
Donor screening strategies and the prevalence of risk factors in the society account for the variation in prevalence rates over time. Currently, the incidence of HBV infection through blood transfusion is relatively low, as screening of blood for transfusion-transmitted infections (TTIs) is the highest priority. However, the residual risk of HBV transmission is associated with the preseroconversion window period and the presence of occult HBV infection, which is characterized by the absence of detectable hepatitis B surface antigen (HBsAg) (5,6). The global incidence of occult hepatitis among blood donors ranges from 0.006% to 17.2% (7).
The high demand for blood transfusion warrants continuous monitoring of the magnitude of transfusion-transmitted infections in blood donors (2). The screening of TTIs in blood centers not only reduces the risk of HBV transmission through transfusion but also provides insight into the prevalence of infection in the healthy population.
The present study was conducted to determine the trend of HBsAg seropositivity among the blood donor population during a five-year period and to demonstrate the difference in prevalence of HBsAg among voluntary and replacement donors. Replacement donors are one-time blood donors who donate blood only when a relative is in need of blood.
Methods
This study was conducted at a licensed blood center of a tertiary care hospital in Bengaluru, India. A retrospective review of blood donor data from January 2018 to December 2022 was extracted. The annual total blood donors were classified into voluntary and replacement donors. All donated blood was screened for the presence of HBsAg using a commercially available ELISA kit (Qualisa, Microwell Enzyme Immunoassay, Qualpro Diagnostics, India). All initially positive samples were retested, and the repeatedly reactive samples were labelled seropositive. According to the National Blood Transfusion Committee policy, positive blood units were discarded, and donors were recalled for counselling and treatment. The data were analyzed for trends in the prevalence of HBsAg. Ethical clearance was obtained from the Institutional Ethics Committee (Ref. no. IEC/A-32/23) before the start of the study, according to the principles of the Declaration of Helsinki.
Results
A total of 18,139 apparently healthy donors were screened during the study period. Among them, 11,517 were replacement donors and 6,622 were voluntary donors, yearly distribution as shown in Figure 1. The overall prevalence of HBsAg seropositivity was 0.66% (Figure 2). The distribution of HBsAg seropositivity among replacement and voluntary donors was 85 and 36, respectively, as depicted in Figure 3. The predominant age group of seropositive donors is shown in Figure 4, with a predominance in the age group of 20 to 40 years. The trends in the seroprevalence of HBsAg during the five-year period are shown in Figure 2, which depict a decline from 0.83% to 0.5%. The lowest seroprevalence was noted in 2022. The annual variation of HBsAg seroprevalence among replacement and voluntary donors is depicted in Figure 3.
 Figure 1. Trend in the distribution of total blood donor population |
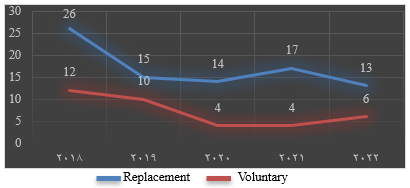 Figure 3. The distribution of HBsAg seropositivity among replacement and voluntary blood donor population |
Discussion
Viral hepatitis is one of the serious complications of blood transfusion, most commonly caused by hepatitis B and hepatitis C viruses. Humans are the only reservoirs of HBV. It is a DNA virus, first discovered by Blumberg et al. in 1965 in the serum of an Australian aborigine (8).
To reduce the risk of transfusion-transmissible diseases, the World Health Organization recommends mandatory blood screening before transfusion (7). The risk of TTIs has declined dramatically in developed countries over the last two decades. However, blood screening in developing countries like India largely depends on ELISA, which targets only HBsAg and cannot detect occult hepatitis, continuing the risk of transfusion-transmitted hepatitis B infection. In recent years, the National Viral Hepatitis Control Programme has been initiated under the National Health Mission of India, for the prevention and control of viral hepatitis, with a view to providing free screening, diagnosis, counselling, and appropriate treatment, especially to people belonging to high-risk groups. Recent advances in diagnostics have now made it possible to diagnose hepatitis infection through point-of-care diagnostic kits. Several new technologies and platforms are also now available for conducting confirmatory tests through viral load testing (9).
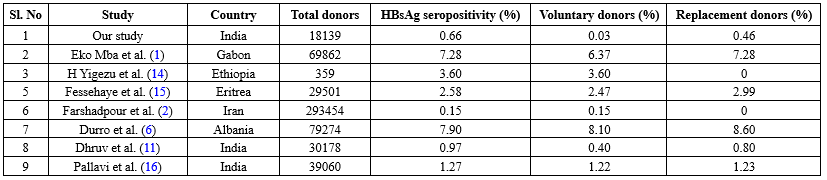
The overall seroprevalence of HBsAg in our study was 0.66%. This was comparable to studies done by Singh et al. (0.62%) (10) and Dhruva G. A. et al. (0.97%) (11). However, a higher prevalence was noted in studies done in Rajasthan (3.44%) (12) and Delhi (2.22%) (13) (Figure 2), while the prevalence in other geographic areas is tabulated in Table 1. All the studies done across India show a higher prevalence of HBsAg among replacement donors, as the replacement method of blood collection was widely practiced until recent years. The variation in prevalence among different studies is probably due to differences in social behavior, lifestyle, socioeconomic status, immunization status, and the level of awareness regarding the infection.
The majority of donors (63%) in our study were replacement donors. Replacement donors are one-time blood donors who donate blood only when a relative is in need of blood, while voluntary donors are motivated blood donors who donate at regular intervals. Studies have shown that replacement donors constitute the largest group of blood donors in India (14-16), reflecting a lack of public awareness. For the same reason, the incidence of HBsAg seropositivity was higher in replacement donors (0.46%). A similar finding was noted in studies by Eko Mba et al. (7.4%) (1), Fessehaye et al. (2.99%) (15), Durro et al. (8.6%) (6), and Dhruv et al. (0.8%) (11), as tabulated in Table 1. However, in studies done in Iran and Ethiopia, there was no practice of replacement donation, which contributed to a comparatively lower prevalence of HBsAg seropositivity (2,14).
The trends in HBsAg seropositivity have effectively declined from 2018 to 2022 (Figure 5), which can be attributed to the significant reduction in the number of replacement donors. The declining trends may be due to public awareness created through the conduction of blood donation camps. The voluntary blood donors mainly consisted of students, religious groups, and voluntary organizations. Moreover, the National Blood Transfusion Committee of India has implemented strict donor selection criteria and proper counselling of blood donors, contributing to the significant decline in the prevalence of HBsAg among blood donors (9).
A similar decreasing trend has been reported in previous studies by Pallavi et al., India (16); Farshadpour, Iran (2); Ahmed et al., Pakistan (17); Abdullah, Saudi Arabia (18); and Okoroiwu, Nigeria (19). Despite these improvements, zero residual risk transmission of HBsAg remains a distant goal, especially in developing countries, due to difficulties in the implementation of nucleic acid amplification test or polymerase chain reaction as screening tests.
The trends in HBsAg seropositivity have effectively declined from 2018 to 2022 (Figure 5), which can be attributed to the significant reduction in the number of replacement donors. The declining trends may be due to public awareness created through the conduction of blood donation camps. The voluntary blood donors mainly consisted of students, religious groups, and voluntary organizations. Moreover, the National Blood Transfusion Committee of India has implemented strict donor selection criteria and proper counselling of blood donors, contributing to the significant decline in the prevalence of HBsAg among blood donors (9).
A similar decreasing trend has been reported in previous studies by Pallavi et al., India (16); Farshadpour, Iran (2); Ahmed et al., Pakistan (17); Abdullah, Saudi Arabia (18); and Okoroiwu, Nigeria (19). Despite these improvements, zero residual risk transmission of HBsAg remains a distant goal, especially in developing countries, due to difficulties in the implementation of nucleic acid amplification test or polymerase chain reaction as screening tests.
|
Table 1. Comparison of our study with other studies across the globe
 |
Conclusion
Our study shows a declining trend and prevalence of HBsAg seropositivity among blood donors, which is an encouraging sign, reflecting the effectiveness of strict donor selection criteria, the conduction of blood donation camps, and the use of sensitive screening tests. Although seropositivity can be further reduced by implementing nucleic acid amplification test and polymerase chain reaction, these diagnostic tests are financially and technically beyond reach in developing countries like India. To conclude, promoting and encouraging voluntary blood donation is a simple and effective way to reduce the prevalence of all TTIs.
Acknowledgement
We extend our gratitude to the institution and the blood center staff for their invaluable contribution to the study.
Funding sources
None.
Ethical statement
Permission was obtained from the Institutional Ethics Committee.
Conflicts of interest
None.
Author contributions
Yashica Gowda and Suja Ajoy Kumar conceived the presented idea. Yashica Gowda and Karthik Srevatsa verified the analytical methods. Suja Ajoy Kumar encouraged the investigation and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Research Article: Original Paper |
Subject:
Pathology
Received: 2023/12/27 | Accepted: 2024/05/25 | Published: 2025/09/17 | ePublished: 2025/09/17
Received: 2023/12/27 | Accepted: 2024/05/25 | Published: 2025/09/17 | ePublished: 2025/09/17
References
1. Eko Mba JM, Bisseye C, Ntsame Ndong JM, Mombo LE, Bengone C, Mouelet Migolet G, et al. Prevalent hepatitis B surface antigen among first-time blood donors in Gabon. PLoS One. 2018;13(4):e0194285. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and Trends of Transfusion-Transmissible Viral Infections among Blood Donors in South of Iran: An Eleven-Year Retrospective Study. PLoS ONE. 2016;11(6):e0157615. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Fast Facts on Global Hepatitis B Vaccination.Centers for Disease Control and Prevention.2024. [View at Publisher]
4. WHO. Blood safety and availability, WHO fact sheet. 2025. [View at Publisher]
5. Candotti D, Laperche S. Hepatitis B Virus Blood Screening: Need for Reappraisal of Blood Safety Measures? Front Med (Lausanne). 2018;5:29. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Durro V, Qyra S. Trends in prevalence of hepatitis B virus infection among Albanian blood donors, 1999-2009. Virol J. 2011;8:96. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Gemechu G, Abagez WE, Alemayehu DH, Tesfaye A, Tadesse D, Kinfu A, et al. Occult Hepatitis B Virus Infection Among Blood Donors in the Capital City of Addis Ababa, Ethiopia: Implications for Blood Transfusion Safety. Front. Gastroenterol.2022;1:887260. [View at Publisher] [DOI] [Google Scholar]
8. Makroo RN. Principles and Practice of Transfusion Medicine 2nd ed. Transfusion transmitted infections.2018;202-16. [View at Publisher]
9. National viral hepatitis control programme, National Health Mission. Ministry of Health and Family Welfare Goverment of India.2021. [View at Publisher]
10. Singh K, Bhat S, Shastry S. Trend in seroprevalence of Hepatitis B virus infection among blood donors of coastal Karnataka, India. J Infect Dev Ctries. 2009;3(5):376-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Dhruva GA, Agravat AH, Pujara KM. Seroprevalence of HIV, HBV, HCV and Syphilis in Blood Donors in Saurashtra Region of Gujarat: Declining Trends Over a Period of 3½ Years. Online J Health Allied Scs. 2012;11(1):5 [View at Publisher] [Google Scholar]
12. Garg S, Mathur DR, Gard DK. Comparison of seropositivity of HIV, HBV, HCV and syphilis in replacement and voluntary blood donors in western India. Indian J Pathol Microbiol. 2001;44(4):409-12. [View at Publisher] [PMID] [Google Scholar]
13. Pahuja S, Sharma M, Baitha B, Jain M. Prevalence and trends of markers of hepatitis C virus, hepatitis B virus and humany immunodeficiency virus in Delhi blood donors. A hospital based study. Jpn J Inf Dis. 2007;60(6):389-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Yigezu H, Temam J, Bajiro M, Tesfaye Jule L, Nagaprasad N, Roy A, et al. Factors Associated with the Prevalence of Hepatitis B among Volunteer Blood Donors at Jimma Blood Bank, South Ethiopia. Can J Gastroenterol Hepatol. 2022;2022: 7458747. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Fessehaye N, Naik D, Fessehaye T. Transfusion transmitted infections - a retrospective analysis from the National Blood Transfusion Service in Eritrea. Pan Afr Med J. 2011;9: 40. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Pallavi P, Ganesh CK, Jayashree K, Manjunath GV. Seroprevalence and trends in transfusion transmitted infections among blood donors in a university hospital blood bank: a 5 year study. Indian J Hematol Blood Transfus. 2011;27(1):1-6 [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Ahmed R, Fatima M, Ashfaq J, Tariq SF, Naseer I, Asif M, et al. Frequency of Hepatitis B, C, and Human Immunodeficiency Virus in Blood Donors. Cureus. 2022;14(6):e25978. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Abdullah SM. Prevalence of hepatitis B and C in donated blood from the jazan region of saudi arabia. Malays J Med Sci. 2013;20(2):41-6. [View at Publisher] [PMID] [Google Scholar]
19. Okoroiwu HU, Okafor IM, Asemota EA, Okpokam DC. Seroprevalence of transfusion-transmissible infections (HBV, HCV, syphilis, and HIV) among prospective blood donors in a tertiary health care facility in Calabar, Nigeria; an eleven-year evaluation. BMC Public Health. 2018;18,645. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |











 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com