Volume 18, Issue 3 (May-Jun 2024)
mljgoums 2024, 18(3): 8-12 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dwivedi S, Rahangdale V, Bhise S, Zodpey S. An update on the increasing prevalence of multidrug-resistant pathogens found in mechanically ventilated patients in central India. mljgoums 2024; 18 (3) :8-12
URL: http://mlj.goums.ac.ir/article-1-1762-en.html
URL: http://mlj.goums.ac.ir/article-1-1762-en.html
1- Department of Microbiology, Indira Gandhi Government Medical College and Hospital Nagpur, 440018, India , sonakshidwivedi6@gmail.com
2- Department of Microbiology, Government Medical College and Hospital (GMCH) Nagpur, 440003, India
3- Department of Microbiology, Indira Gandhi Government Medical College and Hospital Nagpur, 440018, India
2- Department of Microbiology, Government Medical College and Hospital (GMCH) Nagpur, 440003, India
3- Department of Microbiology, Indira Gandhi Government Medical College and Hospital Nagpur, 440018, India
Keywords: Acinetobacter Baumannii, Ventilator-associated Pneumonia, Endotracheal aspirate, Multidrug-resistant organisms
Full-Text [PDF 513 kb]
(1149 Downloads)
| Abstract (HTML) (4679 Views)
Full-Text: (1006 Views)
Introduction
Mechanical ventilation is a life-restoring approach, still, it is still responsible for acquiring respiratory infections, causing maximum mortality in critical patients (1). Ventilator-associated pneumonia (VAP) depicts infection of the lung parenchyma acquired by the invading pathogens, attained post-ventilation (2). Intensive care unit (ICU) infection is an autonomous foreteller for bad outcomes, and VAP caused by multidrug-resistant (MDR) strains is hard to cure (3). The human microbiome has been known as a pool of antibiotic-resistance genes. Bacteria use a genetic mechanism to resist antibiotic effect via gene mutations linked with antibiotic action and acquire resistant genes via horizontal transfer such as transformation, transduction, and conjugation of plasmids or transposons (4). Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. are known as the “ESKAPE” group of pathogens that have developed multidrug resistance property (5). These bacteria are mainly responsible for life-threatening nosocomial infections in mechanically ventilated patients, characterized by probable drug-resistant procedures (5).
The incidence of MDR isolates differs between healthcare centers and among different patient groups, as a part of patients with complicated infections are expected to have an enormous amount of drug resistance (6). Moreover, comprehending the role of antimicrobial resistance related to VAP is critical in this century of ceaseless progression of resistant clones that pose a dire hazard to universal health (7). Therefore, the present study aims to determine the existing scenario of MDR strains arising in mechanically ventilated patients. This study also aims to screen the presence of β-lactamase producers among the isolates; subsequently, the organisms isolated from endotracheal aspirate causing VAP will be identified.
Methods
This cross-sectional study was conducted for 1.5 years, from May 2021 to October 2022, at the Department of Microbiology, GMCH, Nagpur, after obtaining due approval from our institutional ethics committee with registration number 1444. Patient consent was obtained before the investigation by the hospital administration. The researchers confirm that the present study complies with all the regulations. Clinical trials in the present study were not applicable. The following were the inclusion criteria of the patients taken for this study: (i) The age of the patients was greater than 18 years, (ii) Patients who underwent mechanical ventilation for more than 48 hours, and (iii) Patients fulfilling the radiological and clinical parameters. The radiological and clinical conditions include the existence of any recent or ongoing lung infiltrate, in addition to any two of these traits, including fever more than 38°C, high or low white blood cell count, and purulent lower pulmonary secretions (8).
The present study consisted of all adult patients on mechanical ventilation admitted to the medicine intensive care unit of a tertiary care hospital in central India. A total of 410 endotracheal secretions were collected. The endotracheal aspirate of patients was immediately inoculated and streaked onto nutrient agar, 5 % sheep blood agar, and MacConkey agar (HiMedia Laboratories Pvt. Limited, Mumbai, India). Agar plates were incubated under aerobic conditions at 37°C for 24 hours. Isolated strains were processed and distinguished as per the standard bacteriological procedures, and pathogens were identified based on the results of biochemical test (9).
Antimicrobial susceptibility testing was done using the Kirby-Bauer disc diffusion method (10). Drugs used in the testing were as per recommendations mentioned in Clinical and Laboratory Standards Institute 2021 (11). Each gram-negative isolate was further subjected to extended-spectrum ß-lactamases (ESBL), AmpC (Cephalosporinases), and metallo-beta-lactamase (MBL) production for detection of β-lactamase producers. Furthermore, the gram-positive organisms were tested for methicillin-resistant Staphylococcus aureus and inducible clindamycin resistance. The tests conducted for gram-negative isolates were as follows:
Mechanical ventilation is a life-restoring approach, still, it is still responsible for acquiring respiratory infections, causing maximum mortality in critical patients (1). Ventilator-associated pneumonia (VAP) depicts infection of the lung parenchyma acquired by the invading pathogens, attained post-ventilation (2). Intensive care unit (ICU) infection is an autonomous foreteller for bad outcomes, and VAP caused by multidrug-resistant (MDR) strains is hard to cure (3). The human microbiome has been known as a pool of antibiotic-resistance genes. Bacteria use a genetic mechanism to resist antibiotic effect via gene mutations linked with antibiotic action and acquire resistant genes via horizontal transfer such as transformation, transduction, and conjugation of plasmids or transposons (4). Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. are known as the “ESKAPE” group of pathogens that have developed multidrug resistance property (5). These bacteria are mainly responsible for life-threatening nosocomial infections in mechanically ventilated patients, characterized by probable drug-resistant procedures (5).
The incidence of MDR isolates differs between healthcare centers and among different patient groups, as a part of patients with complicated infections are expected to have an enormous amount of drug resistance (6). Moreover, comprehending the role of antimicrobial resistance related to VAP is critical in this century of ceaseless progression of resistant clones that pose a dire hazard to universal health (7). Therefore, the present study aims to determine the existing scenario of MDR strains arising in mechanically ventilated patients. This study also aims to screen the presence of β-lactamase producers among the isolates; subsequently, the organisms isolated from endotracheal aspirate causing VAP will be identified.
Methods
This cross-sectional study was conducted for 1.5 years, from May 2021 to October 2022, at the Department of Microbiology, GMCH, Nagpur, after obtaining due approval from our institutional ethics committee with registration number 1444. Patient consent was obtained before the investigation by the hospital administration. The researchers confirm that the present study complies with all the regulations. Clinical trials in the present study were not applicable. The following were the inclusion criteria of the patients taken for this study: (i) The age of the patients was greater than 18 years, (ii) Patients who underwent mechanical ventilation for more than 48 hours, and (iii) Patients fulfilling the radiological and clinical parameters. The radiological and clinical conditions include the existence of any recent or ongoing lung infiltrate, in addition to any two of these traits, including fever more than 38°C, high or low white blood cell count, and purulent lower pulmonary secretions (8).
The present study consisted of all adult patients on mechanical ventilation admitted to the medicine intensive care unit of a tertiary care hospital in central India. A total of 410 endotracheal secretions were collected. The endotracheal aspirate of patients was immediately inoculated and streaked onto nutrient agar, 5 % sheep blood agar, and MacConkey agar (HiMedia Laboratories Pvt. Limited, Mumbai, India). Agar plates were incubated under aerobic conditions at 37°C for 24 hours. Isolated strains were processed and distinguished as per the standard bacteriological procedures, and pathogens were identified based on the results of biochemical test (9).
Antimicrobial susceptibility testing was done using the Kirby-Bauer disc diffusion method (10). Drugs used in the testing were as per recommendations mentioned in Clinical and Laboratory Standards Institute 2021 (11). Each gram-negative isolate was further subjected to extended-spectrum ß-lactamases (ESBL), AmpC (Cephalosporinases), and metallo-beta-lactamase (MBL) production for detection of β-lactamase producers. Furthermore, the gram-positive organisms were tested for methicillin-resistant Staphylococcus aureus and inducible clindamycin resistance. The tests conducted for gram-negative isolates were as follows:
- ESBL: Based on the combined disk diffusion method, the test organism was inoculated on a Muller Hinton agar plate in the presence of ceftazidime (30μg) disc alone and with clavulanic acid disc (30/10µg). The discs were arranged on the plate and incubated for 24 hours at 37°C. If the strain showed the zone of inhibition of ceftazidime plus clavulanic acid disc, more than or equal to 5 mm that of ceftazidime disc alone, then the organism was described as ESBL producer (11).
- AmpC: Based on the disc antagonism method, the test organism was inoculated on Muller Hinton agar plate in the presence of any of the combination of Cefotaxime (30 µg) and cefoxitin (30 µg) discs or ceftazidime (30 µg) and imipenem (10 µg) discs. The discs were placed 20 mm apart from the center on the plate and incubated at 37°C for 16-18 hours. Isolates showing “blunting” of the cefotaxime zone of inhibition adjacent to the cefoxitin disc or “blunting” of the ceftazidime zone of inhibition beside the imipenem disc were described as inducible AmpC-producing organism (12).
- MBL: Based on the disc potentiation method, the test organism was inoculated on Muller Hinton agar plate, and two imipenem discs (10μg) were placed at a distance of 20 mm from each other. 5μl of 0.5M (750μg) ethylenediamine tetra acetic acid was dispensed on one disc. The plate was incubated at 35°C for 16-20 hours. When the zone diameter of the antimicrobial drugs tested in conjugation with ethylenediamine tetra-acetic acid was greater than or equal to 5 mm of that of the zone diameter when antimicrobial drugs were tested alone, the organism was confirmed as MBL-producing (13).
The following were the tests conducted for gram-positive isolates:
- Test for detection of methicillin-resistant staphylococcus aureus: cefoxitin disc diffusion method: 30µg of cefoxitin disc was placed on the Muller Hinton agar plate inoculated with the test organism. The plate was incubated at 34°C±1°C aerobically for 16 to 18 hours. The test was reported to be positive if the zone of inhibition of cefoxitin is less than or equal to 21 mm; otherwise, it is negative.
- Test for detection of inducible clindamycin resistance:
15 µg of Erythromycin and 2 µg of Clindamycin discs were placed 15 to 26 mm apart. These discs were incubated at 35°C±2°C aerobically for 16 to 18 hours. The test was considered positive if the flat zone (D-shaped zone) of inhibition of clindamycin was observed; in contrast, it was considered negative if hazy growth around clindamycin within the zone of inhibition was observed, even with the appearance of a D-shaped zone.
The inhibition zones were measured, and the organisms were reported as sensitive or resistant to the antimicrobial agent tested according to Clinical Laboratory Standard Institute 2021 guidelines. The standard American Type Culture Collection strain numbers 25923, 25922, 27853, 13883, and 12457 were used for comparison of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii, respectively, for quality control.
Results
VAP reflects an actual public health issue due to its extended duration of mechanical ventilation in the ICU, which incurs additional expense with an increased chance of morbidity, and, therefore, antibiotics are used in mechanically ventilated patients (14). Usually, the mortality rate ranges between 24 and 50 % under normal VAP cases; however, the mortality rate rises to 76 % in VAP cases with MDR strains (15). Minimizing pathogen circulation and antibiotic resistance patterns results in better antibiotic treatment and care of the patients (16).
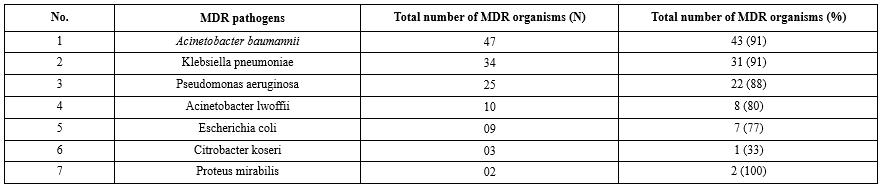
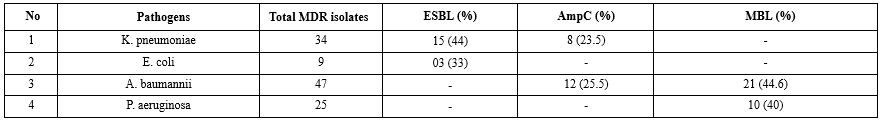
410 endotracheal secretions were received and processed in the microbiology laboratory using standard conventional methods. Out of a total of 410 endotracheal secretions, 332 (81 %) samples showed the presence of bacterial growth. These endotracheal secretions showing growth were further investigated for the symptoms of VAP. Of these 332 samples, 265 (80 %) cases fulfilled the inclusion criteria. 92 (34.7 %) out of 265 samples were confirmed as VAP clinically and microbiologically. The present study reflects the pathogens isolated from VAP primarily A. baumannii (35.6 %) and K. pneumoniae (26 %). Table 1 shows antibiotic resistance pattern for the gram-negative bacilli, depicting maximum resistance against most of the antibiotics (90-100%). Table 2 demonstrate maximum MDR strains in A. baumannii and K. pneumoniae (91%). Table 3 indicates the distribution of β-Lactamases namely ESBL, AmpC and MBL in endotracheal aspirates of VAP cases among MDR isolates.
Discussion
The present study showed that the pathogens isolated from VAP were gram-negative bacilli, predominantly A. baumannii (35.6 %), K. pneumoniae (26 %), and P. aeruginosa (19 %), followed by 7 % of A. lwoffii and E. coli while Proteus mirabilis and Citrobacter koseri were found in traces (1-2 %). Koirala et al. (2010) reported the dominance of gram-negative organisms (40.3%), namely P. aeruginosa, E. coli, Klebsiella spp., and Enterobacter cloacae in tracheal aspirates of 50 patients with fever more than 38°C (17). Tullu et al. (1998) reported the presence of E. coli (33.33%), Klebsiella spp. (29.16%), and Pseudomonas spp. (11.46%) as gram-negative organisms from 70 endotracheal tube tips of patients (18). Nonetheless, S. aureus (23.6%) was the gram-positive organism predominant in the study by Amini et al. (2009). Further, the authors also reported the presence of gram-negative organisms (Klebsiella spp. (23.3%) and Acinetobacter spp. (20.7%)) in the patient’s tracheal tubes in ICU (19).
Table 1 shows non-fermenters' resistance (A. baumannii, P. aeruginosa, and A. lwoffii) against different antibiotics. From Table 1, it can be observed that A. baumannii exhibited more than 95 % resistance against all antibiotics except amikacin. In 2010, Joseph et al. reported 43%, 57%, and 86 % resistance against piperacillin-tazobactam, meropenem, and amikacin, respectively, for A. baumannii, isolated during late-onset VAP (20). Apart from these drugs, the resistance shown against A. baumannii was 100 %. Further, P. aeruginosa showed more than 92 % resistance against tobramycin, levofloxacin, amikacin, cefepime, and piperacillin-tazobactam. Moreover, P. aeruginosa showed 84%, 72%, 64%, and 64% resistance against aztreonam, gentamicin, ceftazidime, and meropenem, respectively. In a study by Goel et al., out of 57 isolates of P. aeruginosa, a high rate of resistance was shown towards aztreonam (94.7%), netilmicin (70.2%), and ceftazidime (68.4%). In comparison, 23 isolates (40%) were resistant to all the other antibiotics used against P. aeruginosa. Meropenem was the most effective (77.2%) drug in vitro, followed by the combination of piperacillin-tazobactam (50.5%) in their study (21). According to a multicentre longitudinal surveillance program, piperacillin-tazobactam and meropenem were the most effective agents against P. aeruginosa, showing resistance of only 20% (22). These findings suggest that meropenem should be used cautiously in ventilated patients to prevent the development of resistance by microorganisms against this drug. It can be predicted that Pseudomonas species show higher levels of antibiotic resistance due to the presence of different types of enzymes (𝛽-lactamases and aminoglycoside-modifying enzymes), the loss of porin proteins and the presence of efflux pumps (23).
Table 1 shows the resistance of Enterobacterales (K. pneumoniae, E. coli, C. koseri, and P. mirabilis) against different antibiotics. From Table 1, it can be observed that K. pneumoniae generated more than 90% resistance against all the antibiotics except gentamicin. Such elevated levels of resistance shown by K. pneumoniae are attributed to the production of ESBL, AmpC, and MBL, along with the efflux of the drug (23). In a case study reported by Dey et al. (2007), 80 % of E. coli and 100 % of K. pneumoniae were ESBL producers; therefore, the authors marked these organisms as MDR (24).
Table 2 shows that the maximum number of MDR pathogens in VAP cases was observed in A. baumannii (91 %) and K. pneumoniae (91 %), followed by P. aeruginosa (88 %), A. lwoffii (80 %), and E. coli (77 %). Traces of C. koseri and P. mirabilis were also found in VAP. A previous study by Kumari et al. (2002) reported the findings from 489 bacterial isolates cultured from lower respiratory tract secretions (Tracheal or bronchoscopy aspirates) of 270 patients in ICU. The authors declared A. baumannii (6.6 %), P. aeruginosa (5 %), and Klebsiella species (1.7 %) as MDR pathogens (25). Furthermore, a study conducted in 2018 reported that 54% K. pneumoniae, 60% A. baumannii, and 19% P. aeruginosa were MDR strains. The study was conducted on 94 respiratory samples obtained from bronchoalveolar lavage (BAL) and endotracheal aspirate (ETA) (26).
In the present study, the high resistance (91 %) shown by A. baumannii against the antibiotics can be attributed to several factors, such as the potential to persist in surrounding and human reservoirs because of less nutritional demand to grow at distinct temperatures and pH, which favor the acquisition of MDR characteristics in A. baumannii. Vila et al. also noted the above observation (27). Further, this study showed that K. pneumoniae had the second highest population of pathogens, which undergoes horizontal transfer of drug-resistant genes through mobile genetic elements that facilitate the production of ESBL and other resistant mechanisms, ultimately aiding the survival of K. pneumoniae in hospitalized patients. Hence, it acquires the MDR characteristics. Ashwath et al. (28) had similar observations regarding K. pneumoniae. P. aeruginosa exhibited MDR properties in the present study and showed a resistance of 88 % against antibiotics, which can be attributed to its innate potential to acquire both inherent and acquired antibiotic resistance from the adjoining bacteria in the surroundings and its capacity to carry multi-resistance plasmids (29). Wagner et al. also described this observation (30). Therefore, it can be concluded that the antibiotic resistance shown by pathogens (A. baumannii, K. pneumoniae, and P. aeruginosa) marked in this study has increased. The rapid emergence of antibiotic-resistant microbes in the ICU could be detrimental to VAP patients.
Table 3 shows that 44 % K. pneumoniae and 33 % E. coli were identified as ESBL producers. Extensive testing of third-generation cephalosporins can be described as a cause for the increase in ESBL producers in India (31). Gram-negative bacteria produce ESBLs that generate resistance against extended-spectrum cephalosporins, aztreonam, narrow-spectrum cephalosporins and anti-gram-negative bacterium penicillins (32). Hirakata et al. (33) identified 11% to 14% of E.coli and Klebsiella species as ESBL producers. A study conducted in Nepal (34) showed a higher prevalence of E. coli at 80% and K. pneumoniae at 57.1%, in contrast to the present study.
Table 3 indicates that 25.5% of A. baumannii and 23.5% of K. pneumoniae were identified as AmpC producers. It can be observed that the above organisms produce plasmid-mediated AmpC β-lactamases; hence, they confer resistance to 7-α-methoxy-cephalosporins (Cefoxitin) and β-lactamase inhibitors (Ampicillin-sulbactam, amoxicillin-clavulanate, and piperacillin-tazobactam). Therefore, these organisms pose new threats to VAP patients. The above observations were also marked by Philippon et al. (35). A study conducted by Golia et al. (36) demonstrated that none of the gram-negative organisms were AmpC producers when the total number of endotracheal aspirate samples was 52. NG et al. (37) examined 98 samples of tracheal aspirates, in which 11 samples were identified as A. baumannii and 19 were identified as K. pneumoniae. Among A. baumannii samples, 9.09 % of isolates were AmpC producers, whereas 10.52 % of isolates of K. pneumoniae samples were AmpC producers.
Table 3 shows that 44.6 % of A. baumannii and 40 % of P. aeruginosa were identified as MBL producers. From the current study, it can be inferred that the gram-negative bacteria producing MBLs can become resistant to carbapenem (Ertapenem and meropenem), making VAP patients difficult to treat. Nordmann et al. (38) reported the above observation in their study. In 2003, a study by Lee et al. (39) reported that MBL production was observed only in 14.2% of A. baumannii and 11.4% of P. aeruginosa. In contrast to the present findings, a previous study in 2015 reported no MBL producers among 11 Acinetobacter spp. (37) and observed 2 (29%) out of 7 P. aeruginosa (40).
Conclusion
The research presented in this paper focused on identifying potential microorganisms as entrants to the multi-drug resistant category. Ventilator-associated pneumonia remains a common challenge for critical patients, leading to significant morbidity, antibiotic use, and costs. The present study presented two important microbiological complications in tracheostomized patients. The first is a high growth rate, and the second is the predominance of multi-drug resistant pathogens, which may be attributed either to selective decontamination of the digestive tract with different antibiotics or empirical use of broad-spectrum antibiotics and non-adherence to hospital antimicrobial position.
A peculiar finding of the present study was that out of 410 endotracheal secretion samples, 80% fulfilled the inclusion criteria, and 34.7% were confirmed to be ventilated-associated pneumonia. Among them, gram-negative strains were also isolated, amongst which the most predominant microorganism was A. baumannii (35.6 %), followed by K. pneumoniae (26 %) and P. aeruginosa (19 %). Among the VAP strains, 80% were multi-drug resistant owing to the production of β‑lactamase such as ESBL (42%), AmpC (24.6%), and MBL (43%), reflecting high resistance rates to common antibiotics such as cephalosporins, carbapenems, aminoglycosides and β-lactam/β-lactamase inhibitor combinations, which are typical and potent drugs for hospital infections leading to high mortality rate.
Recommendation
Making changes to antibiotic prescribing patterns, such as alterations and rotations, could help reduce antibiotic resistance. Moreover, vigilant supervision, laboratory testing of drugs before starting antimicrobial therapy, and implementing a restricted antibiotic policy may improve the management of multidrug-resistant (MDR) pathogens. This can help prevent a scenario similar to the post-antibiotic era, where even common infections may become untreatable, leading to countless deaths.
List of abbreviations
VAP: Ventilator-Associated Pneumonia, ICU: Intensive Care Unit, MDR: Multi-Drug-Resistant, ESBL: Extended-Spectrum 𝛽-Lactamase, MBL: Metallo-𝛽-Lactamases
Acknowledgement
The authors express their gratitude to the Department of Microbiology, GMCH Nagpur, for providing the testing facility essential for conducting the research presented in this paper. Moreover, the authors would like to thank Dr. Waibhaw Kumar (Ph.D., Department of Civil Engineering, Indian Institute of Technology Roorkee) for his expert input in the study.
Funding sources
The research presented in this paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement
Ethical number was obtained after receiving due approval from our institutional ethics committee with registration number 1444.
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Author contributions
Sonakshi Dwivedi: Conceptualization, methodology, data collection, investigation, visualization, writing-original draft, review and editing. Vaishali Rahangdale: Conceptualization and design and data analysis. Swati Bhise: reviewing the initial draft and managing resources. Sunanda Zodpey: project administration and supervising the project. All the authors have critically reviewed and approved the final draft and are responsible for the manuscript's content.
The inhibition zones were measured, and the organisms were reported as sensitive or resistant to the antimicrobial agent tested according to Clinical Laboratory Standard Institute 2021 guidelines. The standard American Type Culture Collection strain numbers 25923, 25922, 27853, 13883, and 12457 were used for comparison of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii, respectively, for quality control.
Results
VAP reflects an actual public health issue due to its extended duration of mechanical ventilation in the ICU, which incurs additional expense with an increased chance of morbidity, and, therefore, antibiotics are used in mechanically ventilated patients (14). Usually, the mortality rate ranges between 24 and 50 % under normal VAP cases; however, the mortality rate rises to 76 % in VAP cases with MDR strains (15). Minimizing pathogen circulation and antibiotic resistance patterns results in better antibiotic treatment and care of the patients (16).
410 endotracheal secretions were received and processed in the microbiology laboratory using standard conventional methods. Out of a total of 410 endotracheal secretions, 332 (81 %) samples showed the presence of bacterial growth. These endotracheal secretions showing growth were further investigated for the symptoms of VAP. Of these 332 samples, 265 (80 %) cases fulfilled the inclusion criteria. 92 (34.7 %) out of 265 samples were confirmed as VAP clinically and microbiologically. The present study reflects the pathogens isolated from VAP primarily A. baumannii (35.6 %) and K. pneumoniae (26 %). Table 1 shows antibiotic resistance pattern for the gram-negative bacilli, depicting maximum resistance against most of the antibiotics (90-100%). Table 2 demonstrate maximum MDR strains in A. baumannii and K. pneumoniae (91%). Table 3 indicates the distribution of β-Lactamases namely ESBL, AmpC and MBL in endotracheal aspirates of VAP cases among MDR isolates.
Discussion
The present study showed that the pathogens isolated from VAP were gram-negative bacilli, predominantly A. baumannii (35.6 %), K. pneumoniae (26 %), and P. aeruginosa (19 %), followed by 7 % of A. lwoffii and E. coli while Proteus mirabilis and Citrobacter koseri were found in traces (1-2 %). Koirala et al. (2010) reported the dominance of gram-negative organisms (40.3%), namely P. aeruginosa, E. coli, Klebsiella spp., and Enterobacter cloacae in tracheal aspirates of 50 patients with fever more than 38°C (17). Tullu et al. (1998) reported the presence of E. coli (33.33%), Klebsiella spp. (29.16%), and Pseudomonas spp. (11.46%) as gram-negative organisms from 70 endotracheal tube tips of patients (18). Nonetheless, S. aureus (23.6%) was the gram-positive organism predominant in the study by Amini et al. (2009). Further, the authors also reported the presence of gram-negative organisms (Klebsiella spp. (23.3%) and Acinetobacter spp. (20.7%)) in the patient’s tracheal tubes in ICU (19).
Table 1 shows non-fermenters' resistance (A. baumannii, P. aeruginosa, and A. lwoffii) against different antibiotics. From Table 1, it can be observed that A. baumannii exhibited more than 95 % resistance against all antibiotics except amikacin. In 2010, Joseph et al. reported 43%, 57%, and 86 % resistance against piperacillin-tazobactam, meropenem, and amikacin, respectively, for A. baumannii, isolated during late-onset VAP (20). Apart from these drugs, the resistance shown against A. baumannii was 100 %. Further, P. aeruginosa showed more than 92 % resistance against tobramycin, levofloxacin, amikacin, cefepime, and piperacillin-tazobactam. Moreover, P. aeruginosa showed 84%, 72%, 64%, and 64% resistance against aztreonam, gentamicin, ceftazidime, and meropenem, respectively. In a study by Goel et al., out of 57 isolates of P. aeruginosa, a high rate of resistance was shown towards aztreonam (94.7%), netilmicin (70.2%), and ceftazidime (68.4%). In comparison, 23 isolates (40%) were resistant to all the other antibiotics used against P. aeruginosa. Meropenem was the most effective (77.2%) drug in vitro, followed by the combination of piperacillin-tazobactam (50.5%) in their study (21). According to a multicentre longitudinal surveillance program, piperacillin-tazobactam and meropenem were the most effective agents against P. aeruginosa, showing resistance of only 20% (22). These findings suggest that meropenem should be used cautiously in ventilated patients to prevent the development of resistance by microorganisms against this drug. It can be predicted that Pseudomonas species show higher levels of antibiotic resistance due to the presence of different types of enzymes (𝛽-lactamases and aminoglycoside-modifying enzymes), the loss of porin proteins and the presence of efflux pumps (23).
Table 1 shows the resistance of Enterobacterales (K. pneumoniae, E. coli, C. koseri, and P. mirabilis) against different antibiotics. From Table 1, it can be observed that K. pneumoniae generated more than 90% resistance against all the antibiotics except gentamicin. Such elevated levels of resistance shown by K. pneumoniae are attributed to the production of ESBL, AmpC, and MBL, along with the efflux of the drug (23). In a case study reported by Dey et al. (2007), 80 % of E. coli and 100 % of K. pneumoniae were ESBL producers; therefore, the authors marked these organisms as MDR (24).
Table 2 shows that the maximum number of MDR pathogens in VAP cases was observed in A. baumannii (91 %) and K. pneumoniae (91 %), followed by P. aeruginosa (88 %), A. lwoffii (80 %), and E. coli (77 %). Traces of C. koseri and P. mirabilis were also found in VAP. A previous study by Kumari et al. (2002) reported the findings from 489 bacterial isolates cultured from lower respiratory tract secretions (Tracheal or bronchoscopy aspirates) of 270 patients in ICU. The authors declared A. baumannii (6.6 %), P. aeruginosa (5 %), and Klebsiella species (1.7 %) as MDR pathogens (25). Furthermore, a study conducted in 2018 reported that 54% K. pneumoniae, 60% A. baumannii, and 19% P. aeruginosa were MDR strains. The study was conducted on 94 respiratory samples obtained from bronchoalveolar lavage (BAL) and endotracheal aspirate (ETA) (26).
In the present study, the high resistance (91 %) shown by A. baumannii against the antibiotics can be attributed to several factors, such as the potential to persist in surrounding and human reservoirs because of less nutritional demand to grow at distinct temperatures and pH, which favor the acquisition of MDR characteristics in A. baumannii. Vila et al. also noted the above observation (27). Further, this study showed that K. pneumoniae had the second highest population of pathogens, which undergoes horizontal transfer of drug-resistant genes through mobile genetic elements that facilitate the production of ESBL and other resistant mechanisms, ultimately aiding the survival of K. pneumoniae in hospitalized patients. Hence, it acquires the MDR characteristics. Ashwath et al. (28) had similar observations regarding K. pneumoniae. P. aeruginosa exhibited MDR properties in the present study and showed a resistance of 88 % against antibiotics, which can be attributed to its innate potential to acquire both inherent and acquired antibiotic resistance from the adjoining bacteria in the surroundings and its capacity to carry multi-resistance plasmids (29). Wagner et al. also described this observation (30). Therefore, it can be concluded that the antibiotic resistance shown by pathogens (A. baumannii, K. pneumoniae, and P. aeruginosa) marked in this study has increased. The rapid emergence of antibiotic-resistant microbes in the ICU could be detrimental to VAP patients.
Table 3 shows that 44 % K. pneumoniae and 33 % E. coli were identified as ESBL producers. Extensive testing of third-generation cephalosporins can be described as a cause for the increase in ESBL producers in India (31). Gram-negative bacteria produce ESBLs that generate resistance against extended-spectrum cephalosporins, aztreonam, narrow-spectrum cephalosporins and anti-gram-negative bacterium penicillins (32). Hirakata et al. (33) identified 11% to 14% of E.coli and Klebsiella species as ESBL producers. A study conducted in Nepal (34) showed a higher prevalence of E. coli at 80% and K. pneumoniae at 57.1%, in contrast to the present study.
Table 3 indicates that 25.5% of A. baumannii and 23.5% of K. pneumoniae were identified as AmpC producers. It can be observed that the above organisms produce plasmid-mediated AmpC β-lactamases; hence, they confer resistance to 7-α-methoxy-cephalosporins (Cefoxitin) and β-lactamase inhibitors (Ampicillin-sulbactam, amoxicillin-clavulanate, and piperacillin-tazobactam). Therefore, these organisms pose new threats to VAP patients. The above observations were also marked by Philippon et al. (35). A study conducted by Golia et al. (36) demonstrated that none of the gram-negative organisms were AmpC producers when the total number of endotracheal aspirate samples was 52. NG et al. (37) examined 98 samples of tracheal aspirates, in which 11 samples were identified as A. baumannii and 19 were identified as K. pneumoniae. Among A. baumannii samples, 9.09 % of isolates were AmpC producers, whereas 10.52 % of isolates of K. pneumoniae samples were AmpC producers.
Table 3 shows that 44.6 % of A. baumannii and 40 % of P. aeruginosa were identified as MBL producers. From the current study, it can be inferred that the gram-negative bacteria producing MBLs can become resistant to carbapenem (Ertapenem and meropenem), making VAP patients difficult to treat. Nordmann et al. (38) reported the above observation in their study. In 2003, a study by Lee et al. (39) reported that MBL production was observed only in 14.2% of A. baumannii and 11.4% of P. aeruginosa. In contrast to the present findings, a previous study in 2015 reported no MBL producers among 11 Acinetobacter spp. (37) and observed 2 (29%) out of 7 P. aeruginosa (40).
Conclusion
The research presented in this paper focused on identifying potential microorganisms as entrants to the multi-drug resistant category. Ventilator-associated pneumonia remains a common challenge for critical patients, leading to significant morbidity, antibiotic use, and costs. The present study presented two important microbiological complications in tracheostomized patients. The first is a high growth rate, and the second is the predominance of multi-drug resistant pathogens, which may be attributed either to selective decontamination of the digestive tract with different antibiotics or empirical use of broad-spectrum antibiotics and non-adherence to hospital antimicrobial position.
A peculiar finding of the present study was that out of 410 endotracheal secretion samples, 80% fulfilled the inclusion criteria, and 34.7% were confirmed to be ventilated-associated pneumonia. Among them, gram-negative strains were also isolated, amongst which the most predominant microorganism was A. baumannii (35.6 %), followed by K. pneumoniae (26 %) and P. aeruginosa (19 %). Among the VAP strains, 80% were multi-drug resistant owing to the production of β‑lactamase such as ESBL (42%), AmpC (24.6%), and MBL (43%), reflecting high resistance rates to common antibiotics such as cephalosporins, carbapenems, aminoglycosides and β-lactam/β-lactamase inhibitor combinations, which are typical and potent drugs for hospital infections leading to high mortality rate.
Recommendation
Making changes to antibiotic prescribing patterns, such as alterations and rotations, could help reduce antibiotic resistance. Moreover, vigilant supervision, laboratory testing of drugs before starting antimicrobial therapy, and implementing a restricted antibiotic policy may improve the management of multidrug-resistant (MDR) pathogens. This can help prevent a scenario similar to the post-antibiotic era, where even common infections may become untreatable, leading to countless deaths.
List of abbreviations
VAP: Ventilator-Associated Pneumonia, ICU: Intensive Care Unit, MDR: Multi-Drug-Resistant, ESBL: Extended-Spectrum 𝛽-Lactamase, MBL: Metallo-𝛽-Lactamases
Acknowledgement
The authors express their gratitude to the Department of Microbiology, GMCH Nagpur, for providing the testing facility essential for conducting the research presented in this paper. Moreover, the authors would like to thank Dr. Waibhaw Kumar (Ph.D., Department of Civil Engineering, Indian Institute of Technology Roorkee) for his expert input in the study.
Funding sources
The research presented in this paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement
Ethical number was obtained after receiving due approval from our institutional ethics committee with registration number 1444.
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Author contributions
Sonakshi Dwivedi: Conceptualization, methodology, data collection, investigation, visualization, writing-original draft, review and editing. Vaishali Rahangdale: Conceptualization and design and data analysis. Swati Bhise: reviewing the initial draft and managing resources. Sunanda Zodpey: project administration and supervising the project. All the authors have critically reviewed and approved the final draft and are responsible for the manuscript's content.
Research Article: Original Paper |
Subject:
Microbiology
Received: 2023/12/18 | Accepted: 2024/06/18 | Published: 2024/11/12 | ePublished: 2024/11/12
Received: 2023/12/18 | Accepted: 2024/06/18 | Published: 2024/11/12 | ePublished: 2024/11/12
References
1. Chandra D, Laghawe A, Tukaram Prabhu KS. Microbiological Profile and Antimicrobial Sensitivity Pattern of Endotracheal Tube Aspirates of Patients in ICU of a Tertiary Care Hospital in Bhopal, India. Int J Curr Microbiol Appl Sci. 2017; 6(3): 891-5. [View at Publisher] [DOI]
2. Rajesh E, Katragadda R, Ramani CP. Bacteriological profile and antimicrobial resistance pattern of ventilator associated pneumonia in tertiary care hospital. Indian J Microbiol Res. 2021; 8(3): 191-5. [View at Publisher] [DOI]
3. Luo W, Xing R, Wang C. The effect of ventilator-associated pneumonia on the prognosis of intensive care unit patients within 90 days and 180 days. BMC Infect Dis. 2021; 21: 1-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Rice LB. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens : No ESKAPE. J infect dis. 2008; 197(8): 1079-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Santajit S, Indrawattana N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int. 2016; 2016: 1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Sangale A, Bhat V, Kelkar R, Biswas S. Microbiology of ventilator-associated pneumonia in a tertiary care cancer hospital. Indian Journal of Critical Care Medicine. 2021; 25(4): 421-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Alnimr A. Antimicrobial Resistance in Ventilator-Associated Pneumonia : Predictive Microbiology and Evidence- Based Therapy. Infect Dis Ther. 2023; 12(6): 1527-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Klompas M. Ventilator-associated events: What they are and what they are not. Respir Care. 2019; 64(8): 953-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Procop GW, Church DL, Hall GS, Janda WM, Koneman EW, Schreckenberger PC, et al. Koneman's Color Atlas and Textbook of Diagnostic Microbiology. 7th ed. Philadelphia : Wolters Kluwer Health; 2017. [View at Publisher] [Google Scholar]
10. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am J Clin Pathol. 1966; 45(4): 493-6. [View at Publisher] [DOI] [PMID]
11. Lewis II J, Weinstein M, Bobenchik AM, Campeau S, Cullen SK, Galas MF, et al. Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. 2021; 42: 1-325. [View at Publisher]
12. Sanders CC, Sanders WE, Goering R V. In Vitro Antagonism of Beta-Lactam Antibiotics by Cefoxitin. Antimicrob Agents Chemother. 1982; 21(6): 968-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA Disk Method for Differentiation of Metallo-β-Lactamase producing Clinical Isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002; 40(10): 3798-801. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Melsen WG, Rovers MM, Groenwold RHH, Bergmans DCJJ, Camus C, Bauer TT, et al. Articles Attributable mortality of ventilator-associated pneumonia : a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013; 13(8): 665-71. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Chastre J, Fagon JY. State of the Art Ventilator-associated Pneumonia. Am J Respir Crit Care Med. 2002; 165: 867-903. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Waters B, Muscedere J. A 2015 Update on Ventilator-Associated Pneumonia: New Insights on Its Prevention, Diagnosis, and Treatment. Curr Infect Dis Rep. 2015; 17(8): 1-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Koirala P, Bhatta DR, Ghimire P, Pokhrel BM, Devkota U. Bacteriological Profile of Tracheal Aspirates of the Patients Attending Neuro-hospital Nepal. International Journal of Life Sciences. 2010; 4: 60-5. [View at Publisher] [DOI] [Google Scholar]
18. Tullu M, Deshmukh C, Baveja S. Bacterial profile and antimicrobial susceptibility pattern in catheter related nosocomial infections. J Postgrad Med. 1998; 44(1): 7-13. [View at Publisher] [PubMed] [Google Scholar]
19. Amini M, Javanmard A, Davati A, Azimi G. Bacterial Colonization in Tracheal Tubes of ICU Patients. Iran J Pathol. 2009;4(3):123-7. [View at Publisher] [Google Scholar]
20. Joseph NM, Sistla S, Kumar Dutta T, Shankar Badhe A, Rasitha D, Chandra Parija S. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. j infect dev ctries. 2010; 4(4): 218-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Goel N, Chaudhary U, Aggarwal R, Bala K. Antibiotic sensitivity pattern of gram negative bacilli isolated from the lower respiratory tract of ventilated patients in the intensive care unit. Indian J Crit Care Med. 2009; 13(3): 148-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Goossens H. MYSTIC program: Summary of European data from 1997 to 2000. Diagn Microbiol Infect Dis. 2001; 41(4): 183-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Khanal S, Joshi DR, Bhatta DR, Devkota U, Pokhrel BM. Bacterial Pathogens from Tracheal Aspirates of Intensive Care Unit Patients at National Institute of Neurological and Allied Sciences , Nepal. ISRN Microbiol. 2013; 2013: 1-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months' prospective study. Ann Thorac Med. 2007; 2(2): 52-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Kumari HBV, Nagarathna S, A C. Antimicrobial Resistance Pattern Among Aerobic Gramnegative Bacilli of Lower Respiratory Tract Specimens of Intensive Care Unit Patients in a Neurocentre. Indian J Chest Dis Allied Sci. 2007;49(1):19-27. [View at Publisher] [PubMed] [Google Scholar]
26. V.S. Silpha, Aruna. A Study to Assess the Bacterial Pathogens of Ventilator Associated Pneumonia in the Intensive Care Unit Patients in a Tertiary Care Hospital in South India. Int J Sci Res. 2019; 8(8): 1281-4. [View at Publisher]
27. Vila J, Martí S, Sánchez-Céspedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007; 59(6): 1210-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Ashwath P, Kotian A, Deekshit VK, J. P, Dharnappa Sannejal A. Klebsiella pneumoniae Infections and Antimicrobial Drug Resistance. In: Siddhardha B, Syed A, Dyavaiah M, editors. Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery. 2020; 195-226. [View at Publisher] [DOI]
29. Livermore DM. Multiple Mechanisms of Antimicrobial Resistance in Pseudomonas aeruginosa: Our Worst Nightmare? Clin Infect dis. 2002; 34(5): 634-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Wagner S, Sommer R, Hinsberger S, Lu C, Hartmann RW, Empting M, et al. Novel Strategies for the Treatment of Pseudomonas aeruginosa Infections. J Med Chem. 2016; 59(13): 5929-69. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Jain A, Roy I, Gupta MK, Kumar M, Agarwal SK. Prevalence of extended-spectrum β-lactamase-producing Gram-negative bacteria in septicaemic neonates in a tertiary care hospital. J Med Microbiol. 2003; 52(5): 421-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Jacoby GA, Medeiros AA. More Extended-Spectrum Beta-Lactamases. Antimicrob Agents Chemother. 1991;35(9):1697-704. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Hirakata Y, Matsuda J, Miyazaki Y, Kamihira S, Kawakami S, Miyazawa Y, et al. Regional variation in the prevalence of extended-spectrum β-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002). Diagn Microbiol Infect Dis. 2005; 52(4): 323-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Poudyal S, Bhatta DR, Shakya G, Upadhyaya B, Dumre SP, Buda G, et al. Extended Spectrum â-lactamase producing multidrug resistant clinical bacterial isolates at National Public Health Laboratory. Nepal. Nepal Med Coll J. 2011; 13(1): 34-8. [PubMed] [Google Scholar]
35. Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002; 46(1): 1-11. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Golia S, Sangeetha KT, Vasudha CL. Microbial profile of early and late onset ventilator associated pneumonia in the intensive care unit of a tertiary care hospital in Bangalore, India. J Clin Diagn Res. 2013; 7(11): 2462-6. [View at Publisher] [Google Scholar]
37. Girish N, Rajendran R. Bacteriological Profile of Ventilator Associated Pneumonia in a Tertiary Care Hospital and their Antibiotic Resistance Pattern. Int J Med Microbiol Trop Dis. 2015; 1(1): 1-5. [View at Publisher] [Google Scholar]
38. Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002; 8(6): 321-31. [View at Publisher] [DOI:10.1046/j.1469-0691.2002.00401.x] [PMID] [Google Scholar]
39. Lee K, Gyo Lee W, Uh Y, Yim Ha G, Cho J, Chong Y. VIM-and IMP-Type Metallo-β-lactamase-Producing Pseudomonas spp. and Acinetobacter spp. in Korean Hospitals. Emerg Infect Dis. 2003; 9(7): 868-71. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Patel A, Lakhani S, Khara R. Microbiological profile of Ventilator associated pneumonia at ICU of rural based teaching hospital. Int J Biol Med Res. 2015;6(1):4732-6. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com