Volume 18, Issue 3 (May-Jun 2024)
mljgoums 2024, 18(3): 36-38 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shad M, Pangh A H, Tohidi F. Assessment of parasitic contamination in the soil of public parks Gorgan city. mljgoums 2024; 18 (3) :36-38
URL: http://mlj.goums.ac.ir/article-1-1756-en.html
URL: http://mlj.goums.ac.ir/article-1-1756-en.html
1- Infectious Disease Research Center, Golestan University of Medical Sciences, Gorgan, Iran
2- Department of Parasitology and Mycology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Infectious Disease Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,tohidi66@yahoo.ca
2- Department of Parasitology and Mycology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
3- Infectious Disease Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,
Full-Text [PDF 431 kb]
(1497 Downloads)
| Abstract (HTML) (6617 Views)
Results
Discussion
The epidemiology of soil-transmitted parasites highlights the significance of soil as a crucial source for transmission. This is attributed to the flexibility of Toxoplasma gondii oocysts and other coccidia oocysts against adverse environmental conditions, as well as physical and chemical factors. (16,22). Oocysts are spread in the environment by wind, water, agricultural fertilizers, earthworms, and arthropods. Also, these factors can cause contamination of surface water, soil, food products, fruits and vegetables (18). Therefore, basic environmental materials such as water and soil are important sources of human contamination (19). In the present study, 40% of the soil of the public parks of Gorgan was contaminated with the eggs of Toxocara species. In the study conducted by Ghomashlooyan's in Isfahan, soil contamination with Toxocara eggs was investigated. According to this study, out of 140 samples collected, 40 samples (28.6%) had Toxocara eggs (23). In Heshmat's study, only 14.6% were infected with Toxocara eggs (24). The decrease in percentage can be due to the size and difference of sampling areas because the sampling was not only from parks but also from other places such as hospitals and schools, where stray dogs and cats are not allowed to commute. Also, the sampling time of the present study was performed in the late autumn and winter, while the two above-mentioned studies were done in summer (23,24). Therefore, the percentage of contamination was lower compared to these two studies.
In Zibaei et al. Study, soil contamination of parks with Toxocara eggs was investigated in Khorram Abad City. In this study, samples were collected from 18 parks in this city, and 63.3% of the samples were contaminated (25). Maraghi et al. collected 291 soil samples from 31 parks in Abadan city and its suburbs. According to this study, the rate of soil contamination with Toxocara eggs was reported as 61.2% (26). Due to the fact that the climate of Abadan is hot and humid, it is obvious that the percentage of soil contamination is higher than that of Isfahan, which has a dry climate. In the Khazan et al. Study, the prevalence of Toxocara eggs was investigated in Tehran. In this study, 600 samples were collected from 120 parks, 10% of which were contaminated (27). A study was conducted by Motazadian et al. To determine the prevalence of worm eggs in public places in Shiraz. In this study, 112 samples were collected. Among these samples, seven samples had Toxocara catti eggs, two samples had Ascaris lumbricoides eggs, and three samples had Strongyloides stercoralis larvae. Also, coccidia oocysts were found in four samples. No pollution was observed during the dry season of the year (28). In a study conducted by the researcher and his colleagues on the soil of parks in Isfahan city, out of 140 samples collected from 28 parks in Isfahan, Cryptosporidium oocysts were found in 31 samples (22.14%) (22). In our study, the most common parasite was Toxocara eggs. Toxocariasis is considered an important neglected disease in Iran. In toxocariasis, humans are infected by ingesting infected eggs in the soil or through contaminated hands. Everyone is susceptible to contamination, but children playing in parks are more susceptible to infection than adults. Contamination is mostly through soil contact. Because the eggs need time to incubate in the soil to become infected (29). Berenji investigated soil contamination with Toxocara eggs in Mashhad and Khavaf public parks. In this study, 340 samples were collected from 39 parks in Mashhad and 29 parks in Khavaf, resulting in 9.2% being infected in Mashhad and 11.3% in Khavaf (30). Tavala conducted a study to investigate the abundance of parasites in soil samples of Tehran. In this study, 150 samples were collected and tested by sodium nitrate flotation method and sucrose method. According to the results obtained from the sodium nitrate method, 38.7% Toxocara eggs, 1.7% Isospora, 4.7% nematode larvae, 8.7% Eimeria species, 27% Dicrocelium dendriticum, 2.7% coccidial oocysts, 76.6% soil nematodes, and 10% Cryptosporidium were found (12). In the Pestechian’s study, this was carried out on the intestinal parasites of stray dogs in Isfahan city. 96 stray dog collars were collected from different areas of Isfahan city, of which 9.21% had Toxascaris leonina and 25.6% had Toxocara canis (31). This finding indicates the contamination of stray dogs in the region and the possibility of soil contamination in areas where animals commute. In the current study, the most contamination happened in walking and garbage areas, which actually had the greenest space and are the places where citizens and stray animals commute. Considering the contamination of stray dogs and cats with zoonotic parasites such as Toxocara and Toxoplasma and their movement in the city, especially in parks, it seems necessary to take care of personal hygiene, especially for children who are more exposed to the soil.
Conclusion
The study results highlight the importance of health education. Using compost and sewage for human and animal fertilizers, particularly in city parks for soil enrichment and flower planting, along with proper disposal of waste and sewage, can significantly contribute to the prevention of zoonotic diseases.
Acknowledgement
This research is extracted from Mahsa Shad's thesis with Project code: 112598. This research was funded by Golestan University of Medical Sciences. We are grateful to the Vice-chancellor of Research and Technology of Golestan University of Medical Sciences for the financial support to conduct this research.
Funding sources
This research was funded by Golestan University of Medical Sciences.
Ethical statement
This study was approved by the Ethics Committee of Golestan University of Medical Sciences Gorgan, Iran with ethics code, IR.GOUMS.REC.1400.390.
Conflicts of interest
The authors have no conflict of interest.
Author contributions
Mahsa Shad: Collected samples and conducted experiments; Ayeneh Hagieh Pangh: Performed specific experiments; Farideh Tohidi: Conceptualization, writing, review, editing, methodology, and analysis. All authors read and approved the final manuscript.
Full-Text: (1498 Views)
Introduction
Parasitic infections transmitted through soil are one of the health problems of human societies that infect many people. In 2010, all over the world, 438.9 million people were infected 438.9 million with hookworm, 919 million with Ascaris lumbricoides, and 464.6 million with Trichuris trichura. On the other hand, 67% of soil - transmitted infections occurred in Asian countries (1). These infections have a significant impact on human health and cause significant problems, especially for children (2,3). Many pathogenic parasites live in the soil because the soil is a good environment and a good source of food for these organisms (4,5). The life cycle of many of these parasites takes place in the soil, and their eggs or larvae may remain in the soil for months or even years until contamination occurs through digestive or skin contact (1). Zoonotic parasitic diseases also occur through eating parasite eggs (6). Strongyloides stercoralis is one of the worms that has a free life cycle in the soil and may cause a dangerous disease in humans (7). Infectious human hookworm larvae live in the surface layer of the soil and can enter the human body through contact with healthy skin. Many other parasitic agents such as Ascaris lumbricoides, Trichuris trichura, Echinococcus granulosus, Entamoeba histolytica and Giardia lamblia cysts, Cryptosporidium and Cyclospora oocysts are also found in the soil (8-10). The main way to transmit soil - borne parasites is ingestion or skin contact (8,9). Parasites, as important agents, infect a wide range of livestock, wild animals, and humans and cause disease in world (11). Parasites that are transmitted through the soil are a large group that cause infection during their growth in the soil or through the contact with of the hosts ' skin with the contaminated soil (4). Soil contamination with infectious larvae, parasite eggs, cysts, and oocysts is a serious risk for zoonotic parasitic infections (12). Many factors, such as sample collection time, parasite isolation methods, sample number and volume, and soil moisture or dryness, can effect on infection (13). Isospora belli is one of the most common parasites among that cause diarrhea in immunocompromised patients. This parasite is a coccidian that lives in the digestive tract and causes diarrhea in tropical regions (14). The oocysts of this parasite are excreted immaturely through feces and mature outside. In terms of epidemiology, soil is an important source for the transmission of soil - transmitted parasites (15). Due to the resistance of Toxoplasma gondii oocysts and oocysts of other coccidian to adverse environmental conditions, these oocysts can be present in water or food (16,17). Oocysts are spread in the environment by wind, water, agricultural fertilizers, earthworms, and arthropods. Also, these factors can cause contamination of surface water, soil, food products, fruits, and vegetables (18). Therefore, basic environmental materials such as water and soil are important sources for human contamination (19,20). Many studies have been conducted on the prevalence of parasites in soil samples from different parts of the world (4). However, there is brief epidemiological information about the prevalence of these parasites in soil samples of different regions of Iran. Therefore, this study was conducted to investigate the helminth and protozoan parasitic contamination in the soil of public parks in Gorgan City, located in the north of Iran.
Methods
In this cross-sectional descriptive study, 80 soil samples were collected from 16 public parks in Gorgan City in 2022. From each park, five samples were taken from the flower-making area, children's play area, walking area, and around the sitting area and garbage dump. Five samples were taken from these regions. After being collected from different parts of the parks, the larger soil particles were crushed. Then 20 grams of each soil sample was placed in a 250 ml Erlenmeyer flask and 50 ml of 5% sodium hydroxide was added to it and left in a fixed position for one hour. Then, the samples were mixed with a shaker for 20 minutes. The whole content of the flask was poured into a 50 ml tube. The sample was centrifuged at 1 500 rpm for three minutes to settle the eggs and oocysts in the bottom. The supernatant was discarded and the precipitate was washed three times with distilled water. After the final wash, the precipitate was suspended in saturated sodium nitrate, then the tubes were placed in a fixed position. Gently, so as not to form bubbles, the coverslip was placed on the tubes and kept in a fixed place for 30 minutes. Then, the coverslips were placed on the slide and examined under the microscope with a 40x lens to look for parasites. Acid-fast staining was used to examine Cryptosporidium oocysts. In this way, some of the centrifuged soil from the previous step was mixed and the smear was prepared. After staining this smear, the slide was fixed with methanol and stained according to modified Ziehl-Neelsen’s method (21).
The data obtained from each park and microscopic tests were entered into IBM SPSS version 18 software, and numbers and percentages were used to express qualitative data.Parasitic infections transmitted through soil are one of the health problems of human societies that infect many people. In 2010, all over the world, 438.9 million people were infected 438.9 million with hookworm, 919 million with Ascaris lumbricoides, and 464.6 million with Trichuris trichura. On the other hand, 67% of soil - transmitted infections occurred in Asian countries (1). These infections have a significant impact on human health and cause significant problems, especially for children (2,3). Many pathogenic parasites live in the soil because the soil is a good environment and a good source of food for these organisms (4,5). The life cycle of many of these parasites takes place in the soil, and their eggs or larvae may remain in the soil for months or even years until contamination occurs through digestive or skin contact (1). Zoonotic parasitic diseases also occur through eating parasite eggs (6). Strongyloides stercoralis is one of the worms that has a free life cycle in the soil and may cause a dangerous disease in humans (7). Infectious human hookworm larvae live in the surface layer of the soil and can enter the human body through contact with healthy skin. Many other parasitic agents such as Ascaris lumbricoides, Trichuris trichura, Echinococcus granulosus, Entamoeba histolytica and Giardia lamblia cysts, Cryptosporidium and Cyclospora oocysts are also found in the soil (8-10). The main way to transmit soil - borne parasites is ingestion or skin contact (8,9). Parasites, as important agents, infect a wide range of livestock, wild animals, and humans and cause disease in world (11). Parasites that are transmitted through the soil are a large group that cause infection during their growth in the soil or through the contact with of the hosts ' skin with the contaminated soil (4). Soil contamination with infectious larvae, parasite eggs, cysts, and oocysts is a serious risk for zoonotic parasitic infections (12). Many factors, such as sample collection time, parasite isolation methods, sample number and volume, and soil moisture or dryness, can effect on infection (13). Isospora belli is one of the most common parasites among that cause diarrhea in immunocompromised patients. This parasite is a coccidian that lives in the digestive tract and causes diarrhea in tropical regions (14). The oocysts of this parasite are excreted immaturely through feces and mature outside. In terms of epidemiology, soil is an important source for the transmission of soil - transmitted parasites (15). Due to the resistance of Toxoplasma gondii oocysts and oocysts of other coccidian to adverse environmental conditions, these oocysts can be present in water or food (16,17). Oocysts are spread in the environment by wind, water, agricultural fertilizers, earthworms, and arthropods. Also, these factors can cause contamination of surface water, soil, food products, fruits, and vegetables (18). Therefore, basic environmental materials such as water and soil are important sources for human contamination (19,20). Many studies have been conducted on the prevalence of parasites in soil samples from different parts of the world (4). However, there is brief epidemiological information about the prevalence of these parasites in soil samples of different regions of Iran. Therefore, this study was conducted to investigate the helminth and protozoan parasitic contamination in the soil of public parks in Gorgan City, located in the north of Iran.
Methods
In this cross-sectional descriptive study, 80 soil samples were collected from 16 public parks in Gorgan City in 2022. From each park, five samples were taken from the flower-making area, children's play area, walking area, and around the sitting area and garbage dump. Five samples were taken from these regions. After being collected from different parts of the parks, the larger soil particles were crushed. Then 20 grams of each soil sample was placed in a 250 ml Erlenmeyer flask and 50 ml of 5% sodium hydroxide was added to it and left in a fixed position for one hour. Then, the samples were mixed with a shaker for 20 minutes. The whole content of the flask was poured into a 50 ml tube. The sample was centrifuged at 1 500 rpm for three minutes to settle the eggs and oocysts in the bottom. The supernatant was discarded and the precipitate was washed three times with distilled water. After the final wash, the precipitate was suspended in saturated sodium nitrate, then the tubes were placed in a fixed position. Gently, so as not to form bubbles, the coverslip was placed on the tubes and kept in a fixed place for 30 minutes. Then, the coverslips were placed on the slide and examined under the microscope with a 40x lens to look for parasites. Acid-fast staining was used to examine Cryptosporidium oocysts. In this way, some of the centrifuged soil from the previous step was mixed and the smear was prepared. After staining this smear, the slide was fixed with methanol and stained according to modified Ziehl-Neelsen’s method (21).
Results
The results of this study showed that out of 80 samples collected from 5 districts of Gorgan City parks, 60 samples (75%) were infected with at least one parasite. Toxocara species eggs were the most common soil parasite in 32 samples (40%). Eimeria species oocysts were found in ten samples (12.5%), Strongyloides eggs and larvae in ten samples (12.5%), hookworm eggs and larvae in nine samples (11.25%), Cryptosporidium species oocysts in eight samples (10%), Trichostrongylus eggs in four samples (5%), Giardia cysts in four samples (5%), Hymenolepis nana eggs in four samples (2.5%), Ascaris eggs in one sample (1.25%) and tapeworm eggs were seen in one sample (1.25%). Also, mites were seen in ten samples (12.5%). The highest and lowest places of parasite contamination were in garbage collection places in 14 parks (87.5%) and children's play in eight parks (37.5%). Banovan and KianShahr parks were among the most polluted parks, and all five sampling sites were infected with different parasites. Alangdare Forest Park was the least polluted park (Figure 1, Table 1).
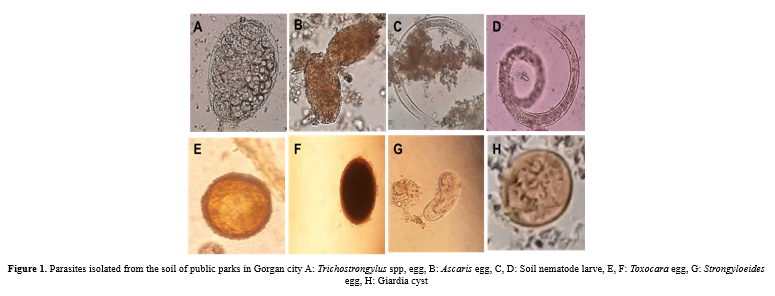 Table 1. Parasite rate of public parks in Gorgan city 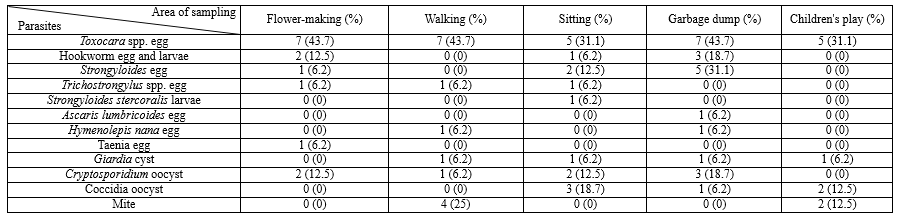 |
Discussion
The epidemiology of soil-transmitted parasites highlights the significance of soil as a crucial source for transmission. This is attributed to the flexibility of Toxoplasma gondii oocysts and other coccidia oocysts against adverse environmental conditions, as well as physical and chemical factors. (16,22). Oocysts are spread in the environment by wind, water, agricultural fertilizers, earthworms, and arthropods. Also, these factors can cause contamination of surface water, soil, food products, fruits and vegetables (18). Therefore, basic environmental materials such as water and soil are important sources of human contamination (19). In the present study, 40% of the soil of the public parks of Gorgan was contaminated with the eggs of Toxocara species. In the study conducted by Ghomashlooyan's in Isfahan, soil contamination with Toxocara eggs was investigated. According to this study, out of 140 samples collected, 40 samples (28.6%) had Toxocara eggs (23). In Heshmat's study, only 14.6% were infected with Toxocara eggs (24). The decrease in percentage can be due to the size and difference of sampling areas because the sampling was not only from parks but also from other places such as hospitals and schools, where stray dogs and cats are not allowed to commute. Also, the sampling time of the present study was performed in the late autumn and winter, while the two above-mentioned studies were done in summer (23,24). Therefore, the percentage of contamination was lower compared to these two studies.
In Zibaei et al. Study, soil contamination of parks with Toxocara eggs was investigated in Khorram Abad City. In this study, samples were collected from 18 parks in this city, and 63.3% of the samples were contaminated (25). Maraghi et al. collected 291 soil samples from 31 parks in Abadan city and its suburbs. According to this study, the rate of soil contamination with Toxocara eggs was reported as 61.2% (26). Due to the fact that the climate of Abadan is hot and humid, it is obvious that the percentage of soil contamination is higher than that of Isfahan, which has a dry climate. In the Khazan et al. Study, the prevalence of Toxocara eggs was investigated in Tehran. In this study, 600 samples were collected from 120 parks, 10% of which were contaminated (27). A study was conducted by Motazadian et al. To determine the prevalence of worm eggs in public places in Shiraz. In this study, 112 samples were collected. Among these samples, seven samples had Toxocara catti eggs, two samples had Ascaris lumbricoides eggs, and three samples had Strongyloides stercoralis larvae. Also, coccidia oocysts were found in four samples. No pollution was observed during the dry season of the year (28). In a study conducted by the researcher and his colleagues on the soil of parks in Isfahan city, out of 140 samples collected from 28 parks in Isfahan, Cryptosporidium oocysts were found in 31 samples (22.14%) (22). In our study, the most common parasite was Toxocara eggs. Toxocariasis is considered an important neglected disease in Iran. In toxocariasis, humans are infected by ingesting infected eggs in the soil or through contaminated hands. Everyone is susceptible to contamination, but children playing in parks are more susceptible to infection than adults. Contamination is mostly through soil contact. Because the eggs need time to incubate in the soil to become infected (29). Berenji investigated soil contamination with Toxocara eggs in Mashhad and Khavaf public parks. In this study, 340 samples were collected from 39 parks in Mashhad and 29 parks in Khavaf, resulting in 9.2% being infected in Mashhad and 11.3% in Khavaf (30). Tavala conducted a study to investigate the abundance of parasites in soil samples of Tehran. In this study, 150 samples were collected and tested by sodium nitrate flotation method and sucrose method. According to the results obtained from the sodium nitrate method, 38.7% Toxocara eggs, 1.7% Isospora, 4.7% nematode larvae, 8.7% Eimeria species, 27% Dicrocelium dendriticum, 2.7% coccidial oocysts, 76.6% soil nematodes, and 10% Cryptosporidium were found (12). In the Pestechian’s study, this was carried out on the intestinal parasites of stray dogs in Isfahan city. 96 stray dog collars were collected from different areas of Isfahan city, of which 9.21% had Toxascaris leonina and 25.6% had Toxocara canis (31). This finding indicates the contamination of stray dogs in the region and the possibility of soil contamination in areas where animals commute. In the current study, the most contamination happened in walking and garbage areas, which actually had the greenest space and are the places where citizens and stray animals commute. Considering the contamination of stray dogs and cats with zoonotic parasites such as Toxocara and Toxoplasma and their movement in the city, especially in parks, it seems necessary to take care of personal hygiene, especially for children who are more exposed to the soil.
Conclusion
The study results highlight the importance of health education. Using compost and sewage for human and animal fertilizers, particularly in city parks for soil enrichment and flower planting, along with proper disposal of waste and sewage, can significantly contribute to the prevention of zoonotic diseases.
Acknowledgement
This research is extracted from Mahsa Shad's thesis with Project code: 112598. This research was funded by Golestan University of Medical Sciences. We are grateful to the Vice-chancellor of Research and Technology of Golestan University of Medical Sciences for the financial support to conduct this research.
Funding sources
This research was funded by Golestan University of Medical Sciences.
Ethical statement
This study was approved by the Ethics Committee of Golestan University of Medical Sciences Gorgan, Iran with ethics code, IR.GOUMS.REC.1400.390.
Conflicts of interest
The authors have no conflict of interest.
Author contributions
Mahsa Shad: Collected samples and conducted experiments; Ayeneh Hagieh Pangh: Performed specific experiments; Farideh Tohidi: Conceptualization, writing, review, editing, methodology, and analysis. All authors read and approved the final manuscript.
Research Article: Research Article |
Subject:
Parasitology
Received: 2023/12/7 | Accepted: 2024/01/3 | Published: 2024/06/26 | ePublished: 2024/06/26
Received: 2023/12/7 | Accepted: 2024/01/3 | Published: 2024/06/26 | ePublished: 2024/06/26
References
1. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & vectors. 2014; 7(1): 1-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Martínez-Moreno F, Hernández S, López-Cobos E, Becerra C, Acosta I, Martínez-Moreno A. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Veterinary parasitology. 2007; 143(1): 7-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Marques JP, Guimarães CdR, Boas AV, Carnaúba PU, Moraes Jd. Contamination of public parks and squares from Guarulhos (São Paulo State, Brazil) by Toxocara spp. and Ancylostoma spp. Revista do Instituto de Medicina Tropical de São Paulo. 2012; 54: 267-71. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Mandarino-Pereira A, de Souza FS, Lopes CWG, Pereira MJS. Prevalence of parasites in soil and dog feces according to diagnostic tests. Veterinary parasitology. 2010; 170(1-2): 176-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends in parasitology. 2003; 19(12): 547-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Ross AG, Olveda RM, McManus DP, Harn DA, Chy D, Li Y, et al. Risk factors for human helminthiases in rural Philippines. International Journal of Infectious Diseases. 2017;54:150-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Arora D, Arora B. Medical Parasitology. New Delhi. Bangalore India. CBS Publishers and Distributors. 2009; 63-66 [View at Publisher]
8. Waenlor W, Wiwanitkit V. Soil examination for soil-transmitted parasite: Importance and experience from Thailand. Journal of Pediatric Infectious Diseases. 2007;2(01): [View at Publisher] [Google Scholar]
9. Davies C, Altavilla N, Krogh M, Ferguson C, Deere D, Ashbolt N. Environmental inactivation of Cryptosporidium oocysts in catchment soils. Journal of Applied Microbiology. 2005; 98(2): 308-17. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Shola K, Majekodunmi R, Adeyinka E. Soil-transmitted helminth infections among school children in rural communities of Moro Local Government Area, Kwara State, Nigeria. African Journal of microbiology research. 2013; 7(45): 5148-53. [View at Publisher] [Google Scholar]
11. Adam R. Biology of Giardia lamblia. Clin Microbiol. Rev. 2001;14(3):447-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Tavalla M, Oormazdi H, Akhlaghi L, Razmjou E, Lakeh MM, Shojaee S, et al. Prevalence of parasites in soil samples in Tehran public places. African Journal of Biotechnology. 2012; 11(20): 4575-8. [View at Publisher] [Google Scholar]
13. Nunes CM, Sinhorini IL, Ogassawara S. Influence of soil texture in the recovery of Toxocara canis eggs by a flotation method. Veterinary Parasitology. 1994;53(3-4):269-74. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Lindsay DS, Dubey J, Blagburn BL. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clinical microbiology reviews. 1997;10(1):19-34. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Aspinall TV, Guy EC, Roberts KE, Joynson DH, Hyde JE, Sims PF. Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: public health implications. International journal for parasitology. 2003;33(1):97-103. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Dubey JP, Jenkins MC, Thayer DW, Kwok OC, Shen SK. Killing of Toxoplasma gondii oocysts by irradiation and protective immunity induced by vaccination with irradiated oocysts. J Parasitol. 1996; 82(5): 724-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Dubey J, Thayer D, Speer C, Shen S. Effect of gamma irradiation on unsporulated and sporulated Toxoplasma gondii oocysts. International Journal for Parasitology. 1998; 28(3): 369-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Ruiz A, Frenkel J. Intermediate and transport hosts of Toxoplasma gondii in Costa Rica. The American Journal of Tropical Medicine and Hygiene. 1980; 29(6): 1161-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Aramini JJ, Stephen C, Dubey J, Engelstoft C, Schwantje H, Ribble C. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiology & Infection. 1999;122(2):305-15. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Sponseller JK, Griffiths JK, Tzipori S. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clinical microbiology reviews. 2014; 27(3): 575-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Gharavi MJ. Laboratory of Clinical Parasitology. 2th ed. Tehran:Timurzadeh Novin;1391. [View at Publisher] [Google Scholar]
22. Mohaghegh MA, Jafari R, Ghomashlooyan M, Mirzaei F, Azami M, Falahati M, et al. Soil contamination with oocysts of Cryptosporidium spp. in Isfahan, Central Iran. International Journal of Enteric Pathogens. 2015;3(3):30-4. [View at Publisher] [DOI] [Google Scholar]
23. Ghomashlooyan M, Falahati M, Mohaghegh MA, Jafari R, Mirzaei F, Kalani H, et al. Soil contamination with Toxocara spp. eggs in the public parks of Isfahan City, Central Iran. Asian Pacific Journal of Tropical Disease. 2015; 5: S93-S5. [View at Publisher] [DOI] [Google Scholar]
24. Heshmat F, Yousefi HA, Tolouei S, Pestehchian N. Prevalence of coccidians and helminthes ova in soil samples from public places in Isfahan City, Iran, 2016. Journal of Isfahan Medical School. 2017; 35(431): 577-82. [View at Publisher] [Google Scholar]
25. Zibaei M, Abdollahpour F, Birjandi M, Firoozeh F. Soil contamination with Toxocara spp. eggs in the public parks from three areas of Khorram Abad, Iran. Nepal med coll J. 2010; 12(2): 63-5. [View at Publisher] [PMID] [Google Scholar]
26. Maraghi S, Mazhab Jafari K, Sadjjadi SM, Latifi SM, Zibaei M. Study on the contamination of Abadan public parks soil with Toxocara spp. eggs. Journal of Environmental Health Science and Engineering. 2014; 12(1): 1-3. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Khazan H, Khazaei M, Tabaee SS, Mehrabi A. Prevalence of Toxocara spp. eggs in public parks in Tehran City, Iran. Iranian journal of parasitology. 2012; 7(3): 38-42. [View at Publisher] [PMID] [Google Scholar]
28. Motazedian H, Mehrabani D, Tabatabaee S, Pakniat A, Tavalali M. Prevalence of helminth ova in soil samples from public places in Shiraz. EMHJ-Eastern Mediterranean Health Journal. 2006; 12 (5), 562-565. [View at Publisher] [PMID] [Google Scholar]
29. Overgaauw PA, Nederland V. Aspects of Toxocara epidemiology: toxocarosis in dogs and cats. Critical reviews in microbiology. 1997;23(3):233-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Berenji F, Movahedi Rudy AG, Fata A, Tavassoli M, Mousavi Bazaz M, Salehi Sangani G. Soil Contamination with Toxocara Spp. Eggs in Public Parks of Mashhad and Khaf, North East of Iran. Iran J Parasitol. 2015; 10(2): 286-9. [View at Publisher] [PMID] [Google Scholar]
31. Pestechian N, Rasouli A, Yoosefi HA. Distribution of Intestinal Worms among Stray Dogs in Isfahan, Iran. Journal of Isfahan Medical School. 2012;29 (173): 2827-33. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com