Volume 18, Issue 4 (Jul-Aug 2024)
mljgoums 2024, 18(4): 8-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Faris Hasan Z, Şay Coşkun U S. Antibiotic resistance profile of Acinetobacter baumannii isolates identified from blood cultures. mljgoums 2024; 18 (4) :8-10
URL: http://mlj.goums.ac.ir/article-1-1692-en.html
URL: http://mlj.goums.ac.ir/article-1-1692-en.html
1- Instutite of Graduate Studies, Tokat Gaziosmanpaşa University, Tokat, Turkey
2- Department of Medical Microbiology, Faculty of Medicine, Tokat Gaziosmanpaşa University, Tokat, Turkey ,umut.saycoskun@gop.edu.tr
2- Department of Medical Microbiology, Faculty of Medicine, Tokat Gaziosmanpaşa University, Tokat, Turkey ,
Full-Text [PDF 408 kb]
(946 Downloads)
| Abstract (HTML) (3626 Views)
Discussion
Positive blood cultures in a patient with systemic indications of infection identify bloodstream infection, which may be secondary to a documented source or primary, meaning without a recognized cause (8). Bloodstream infections are most commonly caused by Gram-negative bacteria, with A. baumannii being one of the most frequent causative agents. In 2023, antibiotic resistance was highlighted in an international prospective observational cohort study (EUROBACT-2) evaluating bloodstream infections in patients hospitalized in intensive care units. A. baumannii was found to be responsible for 20.3% of Gram-negative bloodstream infections, with 84.6% of these isolates being carbapenem-resistant. In addition, 50.3% of the isolates were identified as difficult-to-treat resistant, and 2.3% as pan-drug-resistant (9). In Croatia, the prevalence of MDR A. baumannii strains isolated from bloodstream infections was reported to be 60.2% (10). During the COVID-19 pandemic, A. baumannii was the most frequently isolated bacterium from blood cultures (34%) and showed the highest level of multidrug resistance (100%) among all Gram-negative bacteria (11). In the present study, the prevalence of MDR isolates was found to be 88.9%, a rate comparable to those reported in previous studies. These findings highlight a high prevalence of MDR A. baumannii strains not only in the study area but also globally, emphasizing the importance of identifying the most effective antibiotics for determining appropriate treatment strategies.
Carbapenem-resistant A. baumannii was identified as one of the highest priorities for antibiotic development and research in 2018. Due to its association with a broad spectrum of concurrent resistance to other antibiotic classes, carbapenem resistance has been selected as a marker (12). A significant increase in imipenem resistance among A. baumannii infections was observed from 2011 to 2016, ranging from 73.9% to 77.8%. This resistance rate had dramatically risen compared to the 23.8% reported from 2005 to 2010 in both OECD (Organization for Economic Co-operation and Development) and non-OECD countries (13). Unfortunately, during the 2017-2022 period analyzed, the number of multi-resistant strains of A. baumannii continued to increase, with resistance ranging from 28% to 79% for imipenem and 25% to 76% for meropenem (14). Bagherian et al. reported 90.2% resistance to meropenem and 75% to imipenem (15). Similarly, in the present research, resistance rates of 79.5% to imipenem and 99.1% to meropenem were dedected, which are consistent with recent findings.
Al-Tamimi et al. reported resistance rates of A. baumannii strains to aminoglycosides as 37.2% for tobramycin, 37.1% for amikacin, and 62.6% for gentamicin (16). Jalali et al. reported resistance rates of A. baumannii strains as 85% for tobramycin, 85% for amikacin, and 54% for gentamicin (17). In the present study, resistance to these three antibiotics was observed similarly, with the highest resistance noted for gentamicin (87.2%). Resistance to gentamicin, in particular, has increased significantly in A. baumannii isolates (14).
In Bratislava, resistance rates of 90% for ceftazidime, 85% for cefepime, 90% for cefotaxime, and 100% for cefuroxime among A. baumannii strains have been reported (17). In Jordan, resistance rates of 99.5% for cefoxitin, 77.9% for ceftazidime, and 81.9% for cefepime were observed (16). In the current study, resistance rates of 82.9% for cefoxitin, 94.9% for ceftazidime, and 91.5% for cefepime were noted. High resistance rates were observed for cephalosporins in A. baumannii isolates.
Fluoroquinolones have shown effective activity against A. baumannii isolates over the past four decades. However, resistance to these drugs has rapidly emerged (18). Recent studies conducted in various countries report high rates of resistance to quinolones among A. baumannii isolates (15,17). In the present study, a remarkably high level of resistance to fluoroquinolones was observed, with ciprofloxacin (95.7%) and levofloxacin (94.9%) showing the highest resistance rates after imipenem.
The resistance of A. baumannii isolates to tigecycline has been reported as 7.2% in a study conducted in Jordan (16). Similarly, a study involving hospitalized patients in Brazil reported a resistance rate of 7.1% for A. baumannii isolates (19). Tewari et al. documented a resistance rate of 20% to tigecycline (20), while Tafreshi et al. indicated a resistance rate of 33.3% to tigecycline in 84 MDR A. baumannii isolates (21). In the present study, the resistance rate to tigecycline was determined to be 27.3%. Despite regional variations in resistance rates, tigecycline remains one of the most effective antibiotics against A. baumannii isolates. Consequently, tigecycline may be considered an alternative treatment option for MDR A. baumannii infections.
Escalating MDR isolates have necessitated the use of colistin, which serves as the last-line treatment option for these isolates (22). Farajnia et al. reported that 41.73% of isolates were MDR, with colistin susceptibility at 76% (23). Resistance to colistin has been reported as lower in other studies (4.2% and 10.6%), and it is noteworthy that resistance varies regionally (24,25). In the present investigation, the prevalence of colistin resistance was reported to be 17.1%. Given that the level of resistance observed in the present study was not significantly high, colistin remains a viable treatment option for patients infected with MDR A. baumannii isolates.
Since 2016, EUCAST has recommended broth microdilution (BMD) for determining the minimum inhibitory concentration of colistin (26). However, reference BMD, which requires freshly prepared or frozen antibiotic solutions, is not performed in all clinical laboratories due to laboratory circumstances. Unfortunately, in this study, sensitivity to colistin was determined by the VITEK 2 (bioMérieux, France) automated system and could not be confirmed by BMD. This is a limitation of the study.
Conclusion
Bloodstream infections are a major cause of mortality and morbidity in hospitalized patients worldwide. Increasing rates of antibiotic resistance complicate the treatment of these infections. The growing number of MDR A. baumannii isolates poses a significant threat to hospitalized patients. However, colistin and tigecycline are still considered preferable treatment options for MDR A. baumannii infections. Given the rising number of MDR A. baumannii isolates, periodic analysis of epidemiological data in healthcare centers is crucial to controlling resistance to tigecycline and colistin.
Acknowledgement
Not applicable.
Funding sources
The authors received no financial support for the research, authorship, or publication of this article.
Ethical statement
This study was approved by the Scientific and Ethical Committee of the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (21-KAEK-245).
Conflicts of interest
The authors have stated that they have no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
Author contributions
ZFH, USŞC: Research concept and design; USŞC: Collection and/or assembly of data; ZFH, USŞC: Data analysis and interpretation; ZFH, USŞC: Writing the article; USŞC: Critical revision of the article. All authors read and approved the final version of the article.
Full-Text: (790 Views)
Introduction
Acinetobacter baumannii (A. baumannii) has shown an increasing ability to enhance survival, evade the immune system, and exhibit other virulence characteristics through various determinants such as capsules, outer membrane proteins, biofilms, siderophores, and more (1,2). Acinetobacter spp. are primarily responsible for healthcare-associated infections, including central line-associated bloodstream infections, ventilator-associated pneumonia, and surgical wound infections. Once established, Acinetobacter spp. can persist in healthcare settings and are challenging to eliminate. The most notable rise in cases during the first two years of the COVID-19 pandemic involved carbapenem-resistant Acinetobacter spp. infections, particularly in countries with a relatively high percentage of carbapenem-resistant cases before the pandemic. According to the European Centre for Disease Prevention and Control and the World Health Organization, the percentages of carbapenem-resistant Acinetobacter spp. varied significantly across the region, ranging from below 1% in three (7%) of 45 countries reporting data on this microorganism to 50% or more in 25 (56%) countries in 2021 (3).
A. baumannii has emerged as the predominant etiological agent responsible for bloodstream infections among hospitalized patients. Blood culture, as one of the critical samples analyzed by the clinical microbiology laboratory, serves as the primary and highly sensitive method for diagnosing bloodstream infections. Furthermore, the results of blood cultures play a crucial role in determining the appropriate antimicrobial treatments for patients (4,5).
The clinical properties of bloodstream infections caused by A. baumannii can range from transient and benign bacteremia to severe manifestations, such as fulminant disease and septic shock, with an associated mortality rate of up to 46%. Compared to community-acquired cases, hospital-acquired A. baumannii infections exhibit a distinct clinical syndrome characterized by intense and severe infection (6).
Due to limited treatment options, patients often receive inadequate care, resulting in significant consequences for their health. The objective of this study is to assess the resistance of A. baumannii isolates obtained from blood cultures to antibiotics.
Methods
In this retrospective cohort study, the positive blood culture samples from the records of the Microbiology Laboratory at Tokat Gaziosmanpaşa University Research and Application Hospital were evaluated. A total of 117 samples with A. baumannii-positive blood cultures were identified, excluding repeated samples from the same patient. Blood culture samples were examined in each set of blood culture bottles received at the laboratory using the BacT-Alert 3D system (bioMérieux, Durham, NC, USA). All blood culture bottles were incubated for a duration of five days using the designated system equipment. Blood culture bottles displaying positive growth signals were subjected to Gram staining and inoculated onto blood agar (HiMedia, Türkiye) and eosin methylene blue agar (HiMedia, Türkiye). Microorganism identification and antibiotic susceptibility testing were performed using the VITEK 2 (bioMérieux, France) automated system. The antimicrobial susceptibility tests were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (7).
This study was approved by the Scientific and Ethical Committee of the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (Ethical Number: 21-KAEK-245). The data were statistically analyzed using SPSS Statistical Program Version 21.0 (SPSS Inc., Chicago, Illinois, USA). Mean and standard deviation were used to describe quantitative variables with a normal distribution, while mean and range were used to characterize non-normally distributed data. Qualitative characteristics were described using numbers and percentages.
Results
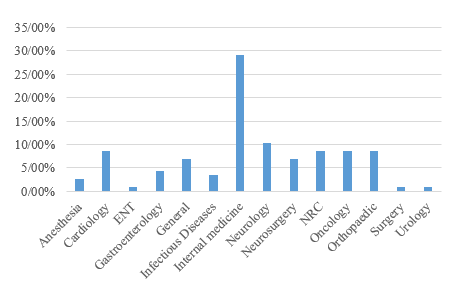
A total of 117 samples were analyzed, of which 59.8% were from male patients and 40.2% from female patients. The average age of the patients was 67.31 ± 15.11 years, with 65.8% being 65 years old or older. The majority of A. baumannii-positive blood culture samples (90.6%) were obtained from patients admitted to the intensive care unit. The distribution of the isolates across different clinics is presented in Figure 1.
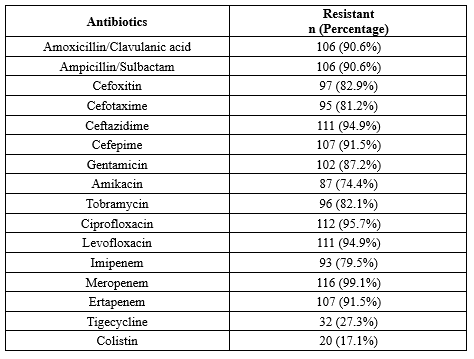
A significant proportion of the A. baumannii isolates (88.9%) were identified as multidrug-resistant (MDR). The majority of MDR A. baumannii isolates (89.4%) were obtained from patient samples taken from intensive care units. The highest resistance was observed to meropenem (99.1%), while the lowest resistance was seen with colistin (17.1%) and tigecycline (27.3%). Resistance to amikacin was 74.4%, whereas resistance levels for gentamicin, tobramycin, cefoxitin, and cefotaxime ranged between 80–90%. Resistance rates for imipenem, amoxicillin/clavulanic acid, ampicillin/sulbactam, ceftazidime, cefepime, ciprofloxacin, levofloxacin, meropenem, and ertapenem exceeded 90%. The antibiotic resistance pattern among A. baumannii isolates from blood cultures is shown in Table 1.
Acinetobacter baumannii (A. baumannii) has shown an increasing ability to enhance survival, evade the immune system, and exhibit other virulence characteristics through various determinants such as capsules, outer membrane proteins, biofilms, siderophores, and more (1,2). Acinetobacter spp. are primarily responsible for healthcare-associated infections, including central line-associated bloodstream infections, ventilator-associated pneumonia, and surgical wound infections. Once established, Acinetobacter spp. can persist in healthcare settings and are challenging to eliminate. The most notable rise in cases during the first two years of the COVID-19 pandemic involved carbapenem-resistant Acinetobacter spp. infections, particularly in countries with a relatively high percentage of carbapenem-resistant cases before the pandemic. According to the European Centre for Disease Prevention and Control and the World Health Organization, the percentages of carbapenem-resistant Acinetobacter spp. varied significantly across the region, ranging from below 1% in three (7%) of 45 countries reporting data on this microorganism to 50% or more in 25 (56%) countries in 2021 (3).
A. baumannii has emerged as the predominant etiological agent responsible for bloodstream infections among hospitalized patients. Blood culture, as one of the critical samples analyzed by the clinical microbiology laboratory, serves as the primary and highly sensitive method for diagnosing bloodstream infections. Furthermore, the results of blood cultures play a crucial role in determining the appropriate antimicrobial treatments for patients (4,5).
The clinical properties of bloodstream infections caused by A. baumannii can range from transient and benign bacteremia to severe manifestations, such as fulminant disease and septic shock, with an associated mortality rate of up to 46%. Compared to community-acquired cases, hospital-acquired A. baumannii infections exhibit a distinct clinical syndrome characterized by intense and severe infection (6).
Due to limited treatment options, patients often receive inadequate care, resulting in significant consequences for their health. The objective of this study is to assess the resistance of A. baumannii isolates obtained from blood cultures to antibiotics.
Methods
In this retrospective cohort study, the positive blood culture samples from the records of the Microbiology Laboratory at Tokat Gaziosmanpaşa University Research and Application Hospital were evaluated. A total of 117 samples with A. baumannii-positive blood cultures were identified, excluding repeated samples from the same patient. Blood culture samples were examined in each set of blood culture bottles received at the laboratory using the BacT-Alert 3D system (bioMérieux, Durham, NC, USA). All blood culture bottles were incubated for a duration of five days using the designated system equipment. Blood culture bottles displaying positive growth signals were subjected to Gram staining and inoculated onto blood agar (HiMedia, Türkiye) and eosin methylene blue agar (HiMedia, Türkiye). Microorganism identification and antibiotic susceptibility testing were performed using the VITEK 2 (bioMérieux, France) automated system. The antimicrobial susceptibility tests were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (7).
This study was approved by the Scientific and Ethical Committee of the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (Ethical Number: 21-KAEK-245). The data were statistically analyzed using SPSS Statistical Program Version 21.0 (SPSS Inc., Chicago, Illinois, USA). Mean and standard deviation were used to describe quantitative variables with a normal distribution, while mean and range were used to characterize non-normally distributed data. Qualitative characteristics were described using numbers and percentages.
Results
A total of 117 samples were analyzed, of which 59.8% were from male patients and 40.2% from female patients. The average age of the patients was 67.31 ± 15.11 years, with 65.8% being 65 years old or older. The majority of A. baumannii-positive blood culture samples (90.6%) were obtained from patients admitted to the intensive care unit. The distribution of the isolates across different clinics is presented in Figure 1.
A significant proportion of the A. baumannii isolates (88.9%) were identified as multidrug-resistant (MDR). The majority of MDR A. baumannii isolates (89.4%) were obtained from patient samples taken from intensive care units. The highest resistance was observed to meropenem (99.1%), while the lowest resistance was seen with colistin (17.1%) and tigecycline (27.3%). Resistance to amikacin was 74.4%, whereas resistance levels for gentamicin, tobramycin, cefoxitin, and cefotaxime ranged between 80–90%. Resistance rates for imipenem, amoxicillin/clavulanic acid, ampicillin/sulbactam, ceftazidime, cefepime, ciprofloxacin, levofloxacin, meropenem, and ertapenem exceeded 90%. The antibiotic resistance pattern among A. baumannii isolates from blood cultures is shown in Table 1.
Discussion
Positive blood cultures in a patient with systemic indications of infection identify bloodstream infection, which may be secondary to a documented source or primary, meaning without a recognized cause (8). Bloodstream infections are most commonly caused by Gram-negative bacteria, with A. baumannii being one of the most frequent causative agents. In 2023, antibiotic resistance was highlighted in an international prospective observational cohort study (EUROBACT-2) evaluating bloodstream infections in patients hospitalized in intensive care units. A. baumannii was found to be responsible for 20.3% of Gram-negative bloodstream infections, with 84.6% of these isolates being carbapenem-resistant. In addition, 50.3% of the isolates were identified as difficult-to-treat resistant, and 2.3% as pan-drug-resistant (9). In Croatia, the prevalence of MDR A. baumannii strains isolated from bloodstream infections was reported to be 60.2% (10). During the COVID-19 pandemic, A. baumannii was the most frequently isolated bacterium from blood cultures (34%) and showed the highest level of multidrug resistance (100%) among all Gram-negative bacteria (11). In the present study, the prevalence of MDR isolates was found to be 88.9%, a rate comparable to those reported in previous studies. These findings highlight a high prevalence of MDR A. baumannii strains not only in the study area but also globally, emphasizing the importance of identifying the most effective antibiotics for determining appropriate treatment strategies.
Carbapenem-resistant A. baumannii was identified as one of the highest priorities for antibiotic development and research in 2018. Due to its association with a broad spectrum of concurrent resistance to other antibiotic classes, carbapenem resistance has been selected as a marker (12). A significant increase in imipenem resistance among A. baumannii infections was observed from 2011 to 2016, ranging from 73.9% to 77.8%. This resistance rate had dramatically risen compared to the 23.8% reported from 2005 to 2010 in both OECD (Organization for Economic Co-operation and Development) and non-OECD countries (13). Unfortunately, during the 2017-2022 period analyzed, the number of multi-resistant strains of A. baumannii continued to increase, with resistance ranging from 28% to 79% for imipenem and 25% to 76% for meropenem (14). Bagherian et al. reported 90.2% resistance to meropenem and 75% to imipenem (15). Similarly, in the present research, resistance rates of 79.5% to imipenem and 99.1% to meropenem were dedected, which are consistent with recent findings.
Al-Tamimi et al. reported resistance rates of A. baumannii strains to aminoglycosides as 37.2% for tobramycin, 37.1% for amikacin, and 62.6% for gentamicin (16). Jalali et al. reported resistance rates of A. baumannii strains as 85% for tobramycin, 85% for amikacin, and 54% for gentamicin (17). In the present study, resistance to these three antibiotics was observed similarly, with the highest resistance noted for gentamicin (87.2%). Resistance to gentamicin, in particular, has increased significantly in A. baumannii isolates (14).
In Bratislava, resistance rates of 90% for ceftazidime, 85% for cefepime, 90% for cefotaxime, and 100% for cefuroxime among A. baumannii strains have been reported (17). In Jordan, resistance rates of 99.5% for cefoxitin, 77.9% for ceftazidime, and 81.9% for cefepime were observed (16). In the current study, resistance rates of 82.9% for cefoxitin, 94.9% for ceftazidime, and 91.5% for cefepime were noted. High resistance rates were observed for cephalosporins in A. baumannii isolates.
Fluoroquinolones have shown effective activity against A. baumannii isolates over the past four decades. However, resistance to these drugs has rapidly emerged (18). Recent studies conducted in various countries report high rates of resistance to quinolones among A. baumannii isolates (15,17). In the present study, a remarkably high level of resistance to fluoroquinolones was observed, with ciprofloxacin (95.7%) and levofloxacin (94.9%) showing the highest resistance rates after imipenem.
The resistance of A. baumannii isolates to tigecycline has been reported as 7.2% in a study conducted in Jordan (16). Similarly, a study involving hospitalized patients in Brazil reported a resistance rate of 7.1% for A. baumannii isolates (19). Tewari et al. documented a resistance rate of 20% to tigecycline (20), while Tafreshi et al. indicated a resistance rate of 33.3% to tigecycline in 84 MDR A. baumannii isolates (21). In the present study, the resistance rate to tigecycline was determined to be 27.3%. Despite regional variations in resistance rates, tigecycline remains one of the most effective antibiotics against A. baumannii isolates. Consequently, tigecycline may be considered an alternative treatment option for MDR A. baumannii infections.
Escalating MDR isolates have necessitated the use of colistin, which serves as the last-line treatment option for these isolates (22). Farajnia et al. reported that 41.73% of isolates were MDR, with colistin susceptibility at 76% (23). Resistance to colistin has been reported as lower in other studies (4.2% and 10.6%), and it is noteworthy that resistance varies regionally (24,25). In the present investigation, the prevalence of colistin resistance was reported to be 17.1%. Given that the level of resistance observed in the present study was not significantly high, colistin remains a viable treatment option for patients infected with MDR A. baumannii isolates.
Since 2016, EUCAST has recommended broth microdilution (BMD) for determining the minimum inhibitory concentration of colistin (26). However, reference BMD, which requires freshly prepared or frozen antibiotic solutions, is not performed in all clinical laboratories due to laboratory circumstances. Unfortunately, in this study, sensitivity to colistin was determined by the VITEK 2 (bioMérieux, France) automated system and could not be confirmed by BMD. This is a limitation of the study.
Conclusion
Bloodstream infections are a major cause of mortality and morbidity in hospitalized patients worldwide. Increasing rates of antibiotic resistance complicate the treatment of these infections. The growing number of MDR A. baumannii isolates poses a significant threat to hospitalized patients. However, colistin and tigecycline are still considered preferable treatment options for MDR A. baumannii infections. Given the rising number of MDR A. baumannii isolates, periodic analysis of epidemiological data in healthcare centers is crucial to controlling resistance to tigecycline and colistin.
Acknowledgement
Not applicable.
Funding sources
The authors received no financial support for the research, authorship, or publication of this article.
Ethical statement
This study was approved by the Scientific and Ethical Committee of the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (21-KAEK-245).
Conflicts of interest
The authors have stated that they have no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
Author contributions
ZFH, USŞC: Research concept and design; USŞC: Collection and/or assembly of data; ZFH, USŞC: Data analysis and interpretation; ZFH, USŞC: Writing the article; USŞC: Critical revision of the article. All authors read and approved the final version of the article.
Research Article: Research Article |
Subject:
Microbiology
Received: 2023/07/13 | Accepted: 2023/11/26 | Published: 2025/03/11 | ePublished: 2025/03/11
Received: 2023/07/13 | Accepted: 2023/11/26 | Published: 2025/03/11 | ePublished: 2025/03/11
References
1. Harding CM, Kinsella RL, Palmer LD, Skaar EP, Feldman MF. Medically Relevant Acinetobacter Species Require a Type II Secretion System and Specific Membrane-Associated Chaperones for the Export of Multiple Substrates and Full Virulence. PLoS Pathog. 2016; 12(1): e1005391. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev. 2017; 30(1): 409-447. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. WHO. Antimicrobial resistance surveillance in Europe 2023 - 2021 data. Stockholm: European Centre for Disease Prevention and Control and World Health Organization; 2023. [View at Publisher]
4. Snyder JW. Blood cultures: The importance of meeting pre-analytical requirements in reducing contamination, optimizing sensitivity of detection, and clinical relevance. Clin Microbiol Newsl. 2015; 37(7): 53-7. [View at Publisher] [DOI] [Google Scholar]
5. Baba S K, Jan A, Lone M S, Kakru D K, Fomda B A, Bashir G, et al . Early Detection of Antibiotic Resistance in Positive Blood Cultures: A Study from a Tertiary Care Center in India. Med Lab J. 2023; 17(3): 8-14. [View at Publisher] [DOI] [Google Scholar]
6. Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect. 2009; 73(2): 143-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Breakpoints tables for interpretation of MICs and zone diameters. EUCAST documents version 8.1. European Committee on Antimicrobial Susceptibility Testing, 2018. [View at Publisher]
8. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and non-central lineassociated Bloodstream Infection). Device-associated Module, BSI. Centers for Disease Control and Prevention, CDC. January 2019. [View at Publisher]
9. Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT, et al. EUROBACT-2 Study Group, ESICM, ESCMID ESGCIP and the OUTCOMEREA Network. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023; 49(2): 178-190. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Dobrović K, Škrobo T, Selec K, Jelić M, Čivljak R, Peršec J, et al. Healthcare-Associated Bloodstream Infections Due to Multidrug-Resistant Acinetobacter baumannii in COVID-19 Intensive Care Unit: A Single-Center Retrospective Study. Microorganisms. 2023; 11(3): 774. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Alcántar-Curiel MD, Huerta-Cedeño M, Jarillo-Quijada MD, Gayosso-Vázquez C, Fernández-Vázquez JL, Hernández-Medel ML, et al. Gram-negative ESKAPE bacteria bloodstream infections in patients during the COVID-19 pandemic. PeerJ. 2023; 11: e15007. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Tacconelli E, Sifakis F, Harbarth S, Schrijver R, van Mourik M, Voss A, et al. Surveillance for control of antimicrobial resistance. Lancet Infect Dis. 2018; 18(3): e99-e106. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Xie R, Zhang XD, Zhao Q, Peng B, Zheng J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg Microbes Infect. 2018; 7(1): 31. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Mączyńska B, Jama-Kmiecik A, Sarowska J, Woronowicz K, Choroszy-Król I, Piątek D, et al. Changes in Antibiotic Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa Clinical Isolates in a Multi-Profile Hospital in Years 2017-2022 in Wroclaw, Poland. J Clin Med. 2023; 12(15): 5020. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Bagherian F, Nikoonejad A, Allami A, Dodangeh S, Yassen L T, Hosienbeigi B. Investigation of Antibiotic Resistance Pattern in Isolated From Urine and Blood Samples of Patients Admitted To the Intensive Care Unit of Velayat Hospital in Qazvin, Iran. Med Lab J. 2021;15(6):31-37. [View at Publisher] [DOI] [Google Scholar]
16. Al-Tamimi M, Albalawi H, Alkhawaldeh M, Alazzam A, Ramadan H, Altalalwah M, et al. Multidrug-Resistant Acinetobacter baumannii in Jordan. Microorganisms. 2022 ;10(5): 849. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Jalali Y, Liptáková A, Jalali M, Payer J. Moving toward Extensively Drug-Resistant: Four-Year Antimicrobial Resistance Trends of Acinetobacter baumannii from the Largest Department of Internal Medicine in Slovakia. Antibiotics. 2023; 12(7): 1200. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Mohammed MA, Ahmed MT, Anwer BE, Aboshanab KM, Aboulwafa MM. Propranolol, chlorpromazine and diclofenac restore susceptibility of extensively drug-resistant (XDR)-Acinetobacter baumannii to fluoroquinolones. PLoS One. 2020; 15(8): e0238195. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Castilho SRA, Godoy CSM, Guilarde AO, Cardoso JL, André MCP, Junqueira-Kipnis AP, et al. Acinetobacter baumannii strains isolated from patients in intensive care units in Goiânia, Brazil: Molecular and drug susceptibility profiles. PLoS One. 2017; 12(5): e0176790. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Tewari R, Chopra D, Wazahat R, Dhingra S, Dudeja M. Antimicrobial Susceptibility Patterns of an Emerging Multidrug Resistant Nosocomial Pathogen: Acinetobacter baumannii. Malays J Med Sci. 2018; 25(3): 129-134. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Tafreshi N, Babaeekhou L, Ghane M. Antibiotic resistance pattern of Acinetobacter baumannii from burns patients: increase in prevalence of blaOXA-24-like and blaOXA-58-like genes. Iran J Microbiol. 2019; 11(6): 502-509. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Seleim SM, Mostafa MS, Ouda NH, Shash RY. The role of pmrCAB genes in colistin-resistant Acinetobacter baumannii. Sci Rep. 2022; 12(1): 20951. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Farajnia S, Lotfi F, Dehnad A, Shojaie M, Raisi R, Rahbarnia L, et al. The molecular characterization of colistin-resistant isolates of Acinetobacter baumannii from patients at intensive care units. Iran J Microbiol. 2022; 14(3): 319-327. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Perovic O, Duse A, Chibabhai V, Black M, Said M, Prentice E, et al.; for GERMS-SA. Acinetobacter baumannii complex, national laboratory-based surveillance in South Africa, 2017 to 2019. PLoS One. 2022; 17(8): e0271355. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Heydarlou MM, Durmaz G, Ibrahi M BMS. Evaluation of sulbactam and colistin/sulbactam efficacy against multiple resistant Acinetobacter baumannii blood isolates. Indian J Med Microbiol. 2022; 40(4): 567-571. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Poirel L, Jayol A, Nordmanna P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017; 30(2): 557-96. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.