Volume 19, Issue 1 (Jan-Feb 2025)
mljgoums 2025, 19(1): 32-35 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ganji S, Mobedi I, Rezamand A, Ardabili F, Yari P, Khanmohammadi M. Enterobius vermicularis as a cause of acute appendicitis in a child with acute lymphocytic leukemia: A case report. mljgoums 2025; 19 (1) :32-35
URL: http://mlj.goums.ac.ir/article-1-1687-en.html
URL: http://mlj.goums.ac.ir/article-1-1687-en.html
Shalaleh Ganji1 

 , Iraj Mobedi2
, Iraj Mobedi2 

 , Azim Rezamand1
, Azim Rezamand1 

 , Farshid Ardabili3
, Farshid Ardabili3 

 , Pooya Yari4
, Pooya Yari4 

 , Majid Khanmohammadi5
, Majid Khanmohammadi5 




 , Iraj Mobedi2
, Iraj Mobedi2 

 , Azim Rezamand1
, Azim Rezamand1 

 , Farshid Ardabili3
, Farshid Ardabili3 

 , Pooya Yari4
, Pooya Yari4 

 , Majid Khanmohammadi5
, Majid Khanmohammadi5 


1- Department of Pediatric, Children’s Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
2- Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical sciences, Tehran, Iran
3- Department of Biology, Faculty of Sciences, University of Maragheh, Maragheh, Iran
4- Department of Medical Biotechnology, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
5- Department of Laboratory Sciences, Mara.C., Islamic Azad University, Marand, Iran ,majid593@gmail.com
2- Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical sciences, Tehran, Iran
3- Department of Biology, Faculty of Sciences, University of Maragheh, Maragheh, Iran
4- Department of Medical Biotechnology, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
5- Department of Laboratory Sciences, Mara.C., Islamic Azad University, Marand, Iran ,
Full-Text [PDF 496 kb]
(1628 Downloads)
| Abstract (HTML) (5456 Views)
Discussion
E. Vermicularis is a parasite of the gastrointestinal tract with high prevalence in children. Infections caused by E. vermicularis can occur worldwide and are considered one of the extremely contagious infectious diseases (17). This disease occurs in all age groups and social and economic classes, but is more common in children aged 5 to 14 years and in areas with poor health (14,18). Infections caused by this parasite are more common in immunocompromised patients (Such as those with AIDS) or in people taking immunosuppressive drugs (In organ transplantation or autoimmune diseases). For the first time, the presence of enterobiasis in the appendix lumen was reported in the late 19th century (1899) (19). In some studies, acute or chronic inflammatory findings were observed in appendix specimens containing E. vermicularis (14). This parasite infection can cause a clinical picture similar to acute appendicitis due to a hypersensitivity reaction in the tissues (20). According to the worldwide records, Dalimi et al. examined 1590 patients undergoing appendectomy for the presence or absence of E. vermicularis. They were infected with parasites in 38 cases (2.39%), and the highest percentage of infections was observed in females aged 0-9 years (21). In a study conducted by Da Silva et al. in Brazilian health centers, 1600 patients who underwent surgical treatment were examined. It showed that 23 cases (1.43%) were infected with parasites, and the highest percentage of infections occurred in the male age group of less than ten years (7). Siavoshi et al. studied 5314 patients who underwent appendectomy in general hospitals in Hamadan, and it was found that 66 cases (24.1%) were infected with E. vermicularis (22). In 2005, a study was conducted by Yildirim et al. in Adana Hospital in which 104 patients that were surgically treated were examined and it was determined that 4 (8.3%) cases were infected with pinworm (18). In a study by Isik and colleagues of 890 cases of appendectomy, 18 cases (2%) were infected with E. vermicularis (23). Also, in a study by Aydin et al. conducted in Alanya Hospital, 190 cases of appendectomy were studied, and it was found that four cases (2.1%) were infected with E. vermicularis (24). According to Gutierrez (2000), a statistically significant association between E. vermicularis infection and urinary tract infections has been demonstrated (25). However, Burkhart (2005) claims that the incidence of E. vermicularis as a cause of urinary tract infections has not yet been proven (26). In addition, one report indicated that 36% of young girls with a UTI also had painful urination associated with that infection (26). The association between enterobiasis and appendicitis has been documented. However, there is no clear consensus on this issue. While Gutierres (2000) claims that there is a consensus that E. vermicularis cannot cause inflammation of the intestine (25), Cook believes that appendicitis is caused by this worm (27), and Burkhart (2004) states that infection with E. vermicularis causes symptoms of appendicitis (26). Hamood reported a case of acute appendicitis caused by E. vermicularis in a 23-year-old homemaker from Iraq in 2019. E. vermicularis can colonize the appendix and cause symptoms with or without actual histopathological acute appendicitis (28). The belief that E. vermicularis infestation can cause diseases such as acute or chronic appendicitis or perforation of the appendix has been documented (29). However, there are also reports of completely asymptomatic patients (30). All these cases prove that there is a connection between the inflammation of the appendix and even ALL, which is also due to the weakening of the immune system in these cases. Based on the results of previous studies and the importance of this parasite due to its effects on growth, health, nutritional status, and cognitive development (Especially in children), it is proposed that in order to control and prevent the disease, families should be given the necessary information about the life cycle, transmission, and prevention of infection with enterobiasis. In addition, since the infection caused by this parasite is easily transmissible, training should be provided to individuals, and personal and public health care should be improved to avoid the risk of infection with the parasite. Basic measures should also be taken to improve the economic situation of people. It is also recommended that public and private health promotion be considered through education.
Conclusion
According to the results of this report and other studies on this subject, it has been established that infection with this nematode may be associated with inflammation of the appendix or ALL. This is also true in cases where the immune system is weakened (e.g., in AIDS or congenital immunodeficiency syndromes) or when taking immunosuppressants (Special drugs for organ transplants). Therefore, a pinworm screening program remains necessary for immunocompromised patients or those receiving immunosuppressive medications.
Acknowledgement
The authors thank Dr. Davood Badbarin for his valuable suggestions and all the colleagues at the Children’s Hospital of Tabriz.
Funding sources
This study did not receive any funding.
Ethical statement
Since this manuscript is an unusual case report that does not violate the principles of ethics, ethical certification was not required. Therefore, the information's results were published in accordance with the principle of confidentiality.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
Case report: AR and SHGH; data collection: FA, MKH, and AR; data analysis: MKH; draft manuscript: MKH; intellectual contribution and manuscript revision: MKH. All authors read the manuscript and approved its final version.
Data availability statement
All data in this study is publicly available.
Full-Text: (1112 Views)
Introduction
Enterobius vermicularis (Oxyuris-E. vermicularis) is one of the most common worm infections in humans, which can be asymptomatic or symptomatic (1,2). This parasite can cause infections in up to 20% of children (3-5) and can cause appendicitis in up to 4% (4,6-8). This worm is a common cause of parasitic infections in young children (5-10 years) living in confined spaces or nurseries (9). This infection may cause some symptoms in patients, such as anal itching, moodiness, perianal itching, inflammation of the urethra and vagina, insomnia, whimpering, weight loss, and malaise (2). However, one-third of infections do not present with symptoms (2). It is estimated that 200 million people are infected worldwide, including 30-40 million in the United States and Canada (10). The life cycle of adult E. vermicularis is 3-6 weeks, so drug treatment with perfect hygiene can stop the infection cycle (10). E. vermicularis normally lives in the terminal ileum, cecum, vermiform appendix, and proximal part of the colon (11). After fertilization, the female worm migrates to the anus, where oviposition occurs (At night), while the male worm dies (11). Intolerable scratching transfers the eggs to the fingers, and after contamination of the hands, the eggs may enter the mouth. The larvae are released at the beginning of the small intestine and continue their journey toward the appendix, and the worm begins to multiply again (11). In more than 80% of cases in children, anal itching is due to contamination with the parasites (12). When eggs and lifeless parasites enter ectopic sites, granulomas and abscesses may develop (13). E. vermicularis has been poorly detected in the vagina, uterus, and fallopian tubes because the female worm moves around to lay eggs, so this worm sometimes causes vaginitis (10). The Scotch tape test is the standard golden method to detect this worm infection (2). For many years, the relationship between E. vermicularis and appendicitis has been studied, but the relationship between this worm and inflammation is still unknown. Aschoff offered that this parasite causes signs similar to true appendicitis or appendiceal colic (11). For the first time, E. vermicularis was described in the appendix by Fabrius in 1634 (11). In addition, the incidence of appendiceal infections caused by E. vermicularis has been reported to range from 0.65% to 4.1% (4,7,8,14,15). This nematode normally lives in the lumen of the appendix (16). E. vermicularis infections have been shown to be more associated with chronic inflammation (8.3%) of infected appendices compared to appendices without pathological changes (7.0%) or with acute inflammation (0.5%) (4).
Case presentation
The patient was an 8-year-old boy living in Miandoab, West Azerbaijan, Iran. He was admitted to the oncology department of Tabriz Children's Hospital, complaining of fever of 39-40°C with crampy abdominal pain, loss of appetite, weakness, and fatigue, associated with cervical lymphadenopathy, masses in the axillae and thighs, maculopapular rashes, and tiny petechiae on the chest and abdomen, which were found to be hepatosplenomegaly on clinical examination. In laboratory tests, the patient's biochemical and haematological values were as follows (Table 1).
Urine analysis and urine culture were normal. On chest radiography, the mediastinum was wide. Medium mediastinal lymphadenopathy was noted on CT chest radiography. Ultrasonographic examination of the abdomen measured the liver and spleen to be 147 mm and 110 mm, respectively, and concomitant aortic and mesenteric lymphadenopathy was noted. Lymphoblastic cells showed up to 90%, and in flow cytometric examination of bone marrow, CD13, CD10, CD19, CD33, and CD22 were negative, while CD7 was 83%, CD3 was 70%, and HLA-DR was 70%. The patient was hospitalized with a definitive diagnosis of acute lymphoblastic leukemia (ALL). On the seventh day of hospitalization, the patient developed vomiting and anorexia and also developed colic pain (Contraction and cramps) around the umbilicus and concomitant pain in the lower and right abdomen. Clinical examination revealed right iliac fossa tenderness on palpation and rebound tenderness on release. On examination of the right abdomen and lower abdomen, generalized abdominal pain was present (Indicating spread of inflammation to the peritoneum). Based on the signs and laboratory findings, a diagnosis of acute appendicitis was considered for the patient, who underwent surgery. After consultation with the oncologist of the ward and in agreement with the surgeon, the child underwent appendectomy. Because the history on admission to the hospital noted excessive night sweating with anal itching and scratching and increased salivary secretions, the patient was examined with the scotch tape test. On clinical examination, erythematous lesions were noted on the skin around the anus and perianal erythema. In the pathological sections of the appendix, acute inflammation of the appendix was observed along with the cross-section of an adult female E. vermicularis with morphological features such as a uterus containing eggs and the presence of cephalic alae in the head region of E. vermicularis (Figure 1). The patient, with a final diagnosis of enterobiasis, was treated with mebendazole at a dosage of 100 mg twice daily for 3 days, which decreased the gastrointestinal symptoms and anal itching and also improved the patient's general condition. After surgery, the patient was treated with intravenous 400 mg clindamycin, intravenous 500 mg ceftazidime, and clindamycin tablets 400 mg every 12 hours for 4 days. During drug induction, the patient was treated with 60 mg prednisolone as a single dose and 1.5 mg/m² IV vincristine over one minute per week for 5 times. Moreover, the patient received doxorubicin 45 mg/m² on the first day. After injection of 6000 IU/m L-asparaginase on the ninth day, the patient went into remission. With daily 6-mercaptopurine, weekly methotrexate, and monthly vincristine, the patient was discharged in a good and stable general condition.
Enterobius vermicularis (Oxyuris-E. vermicularis) is one of the most common worm infections in humans, which can be asymptomatic or symptomatic (1,2). This parasite can cause infections in up to 20% of children (3-5) and can cause appendicitis in up to 4% (4,6-8). This worm is a common cause of parasitic infections in young children (5-10 years) living in confined spaces or nurseries (9). This infection may cause some symptoms in patients, such as anal itching, moodiness, perianal itching, inflammation of the urethra and vagina, insomnia, whimpering, weight loss, and malaise (2). However, one-third of infections do not present with symptoms (2). It is estimated that 200 million people are infected worldwide, including 30-40 million in the United States and Canada (10). The life cycle of adult E. vermicularis is 3-6 weeks, so drug treatment with perfect hygiene can stop the infection cycle (10). E. vermicularis normally lives in the terminal ileum, cecum, vermiform appendix, and proximal part of the colon (11). After fertilization, the female worm migrates to the anus, where oviposition occurs (At night), while the male worm dies (11). Intolerable scratching transfers the eggs to the fingers, and after contamination of the hands, the eggs may enter the mouth. The larvae are released at the beginning of the small intestine and continue their journey toward the appendix, and the worm begins to multiply again (11). In more than 80% of cases in children, anal itching is due to contamination with the parasites (12). When eggs and lifeless parasites enter ectopic sites, granulomas and abscesses may develop (13). E. vermicularis has been poorly detected in the vagina, uterus, and fallopian tubes because the female worm moves around to lay eggs, so this worm sometimes causes vaginitis (10). The Scotch tape test is the standard golden method to detect this worm infection (2). For many years, the relationship between E. vermicularis and appendicitis has been studied, but the relationship between this worm and inflammation is still unknown. Aschoff offered that this parasite causes signs similar to true appendicitis or appendiceal colic (11). For the first time, E. vermicularis was described in the appendix by Fabrius in 1634 (11). In addition, the incidence of appendiceal infections caused by E. vermicularis has been reported to range from 0.65% to 4.1% (4,7,8,14,15). This nematode normally lives in the lumen of the appendix (16). E. vermicularis infections have been shown to be more associated with chronic inflammation (8.3%) of infected appendices compared to appendices without pathological changes (7.0%) or with acute inflammation (0.5%) (4).
Case presentation
The patient was an 8-year-old boy living in Miandoab, West Azerbaijan, Iran. He was admitted to the oncology department of Tabriz Children's Hospital, complaining of fever of 39-40°C with crampy abdominal pain, loss of appetite, weakness, and fatigue, associated with cervical lymphadenopathy, masses in the axillae and thighs, maculopapular rashes, and tiny petechiae on the chest and abdomen, which were found to be hepatosplenomegaly on clinical examination. In laboratory tests, the patient's biochemical and haematological values were as follows (Table 1).
Urine analysis and urine culture were normal. On chest radiography, the mediastinum was wide. Medium mediastinal lymphadenopathy was noted on CT chest radiography. Ultrasonographic examination of the abdomen measured the liver and spleen to be 147 mm and 110 mm, respectively, and concomitant aortic and mesenteric lymphadenopathy was noted. Lymphoblastic cells showed up to 90%, and in flow cytometric examination of bone marrow, CD13, CD10, CD19, CD33, and CD22 were negative, while CD7 was 83%, CD3 was 70%, and HLA-DR was 70%. The patient was hospitalized with a definitive diagnosis of acute lymphoblastic leukemia (ALL). On the seventh day of hospitalization, the patient developed vomiting and anorexia and also developed colic pain (Contraction and cramps) around the umbilicus and concomitant pain in the lower and right abdomen. Clinical examination revealed right iliac fossa tenderness on palpation and rebound tenderness on release. On examination of the right abdomen and lower abdomen, generalized abdominal pain was present (Indicating spread of inflammation to the peritoneum). Based on the signs and laboratory findings, a diagnosis of acute appendicitis was considered for the patient, who underwent surgery. After consultation with the oncologist of the ward and in agreement with the surgeon, the child underwent appendectomy. Because the history on admission to the hospital noted excessive night sweating with anal itching and scratching and increased salivary secretions, the patient was examined with the scotch tape test. On clinical examination, erythematous lesions were noted on the skin around the anus and perianal erythema. In the pathological sections of the appendix, acute inflammation of the appendix was observed along with the cross-section of an adult female E. vermicularis with morphological features such as a uterus containing eggs and the presence of cephalic alae in the head region of E. vermicularis (Figure 1). The patient, with a final diagnosis of enterobiasis, was treated with mebendazole at a dosage of 100 mg twice daily for 3 days, which decreased the gastrointestinal symptoms and anal itching and also improved the patient's general condition. After surgery, the patient was treated with intravenous 400 mg clindamycin, intravenous 500 mg ceftazidime, and clindamycin tablets 400 mg every 12 hours for 4 days. During drug induction, the patient was treated with 60 mg prednisolone as a single dose and 1.5 mg/m² IV vincristine over one minute per week for 5 times. Moreover, the patient received doxorubicin 45 mg/m² on the first day. After injection of 6000 IU/m L-asparaginase on the ninth day, the patient went into remission. With daily 6-mercaptopurine, weekly methotrexate, and monthly vincristine, the patient was discharged in a good and stable general condition.
|
Table 1. Results of clinical and laboratory parameters
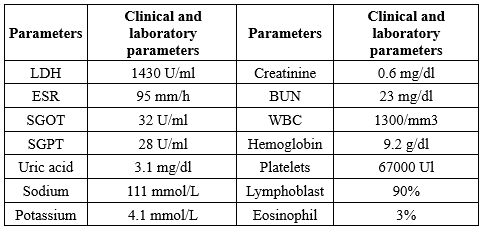 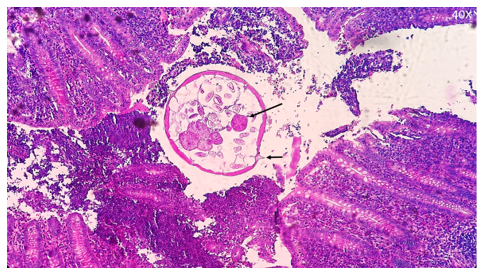 Figure 1. The characteristics of adult E. vermicularis in cross-section in the appendix, stained with hematoxylin/eosin staining (40×). Sectional view of female worm; large arrows indicate the muscular part of the esophagus. The short arrow indicates the cross-section of the expanded multi-layered cuticle and lateral cephalic alea. A distended uterus with intra-uterine eggs and sections of ovaries was seen. |
Discussion
E. Vermicularis is a parasite of the gastrointestinal tract with high prevalence in children. Infections caused by E. vermicularis can occur worldwide and are considered one of the extremely contagious infectious diseases (17). This disease occurs in all age groups and social and economic classes, but is more common in children aged 5 to 14 years and in areas with poor health (14,18). Infections caused by this parasite are more common in immunocompromised patients (Such as those with AIDS) or in people taking immunosuppressive drugs (In organ transplantation or autoimmune diseases). For the first time, the presence of enterobiasis in the appendix lumen was reported in the late 19th century (1899) (19). In some studies, acute or chronic inflammatory findings were observed in appendix specimens containing E. vermicularis (14). This parasite infection can cause a clinical picture similar to acute appendicitis due to a hypersensitivity reaction in the tissues (20). According to the worldwide records, Dalimi et al. examined 1590 patients undergoing appendectomy for the presence or absence of E. vermicularis. They were infected with parasites in 38 cases (2.39%), and the highest percentage of infections was observed in females aged 0-9 years (21). In a study conducted by Da Silva et al. in Brazilian health centers, 1600 patients who underwent surgical treatment were examined. It showed that 23 cases (1.43%) were infected with parasites, and the highest percentage of infections occurred in the male age group of less than ten years (7). Siavoshi et al. studied 5314 patients who underwent appendectomy in general hospitals in Hamadan, and it was found that 66 cases (24.1%) were infected with E. vermicularis (22). In 2005, a study was conducted by Yildirim et al. in Adana Hospital in which 104 patients that were surgically treated were examined and it was determined that 4 (8.3%) cases were infected with pinworm (18). In a study by Isik and colleagues of 890 cases of appendectomy, 18 cases (2%) were infected with E. vermicularis (23). Also, in a study by Aydin et al. conducted in Alanya Hospital, 190 cases of appendectomy were studied, and it was found that four cases (2.1%) were infected with E. vermicularis (24). According to Gutierrez (2000), a statistically significant association between E. vermicularis infection and urinary tract infections has been demonstrated (25). However, Burkhart (2005) claims that the incidence of E. vermicularis as a cause of urinary tract infections has not yet been proven (26). In addition, one report indicated that 36% of young girls with a UTI also had painful urination associated with that infection (26). The association between enterobiasis and appendicitis has been documented. However, there is no clear consensus on this issue. While Gutierres (2000) claims that there is a consensus that E. vermicularis cannot cause inflammation of the intestine (25), Cook believes that appendicitis is caused by this worm (27), and Burkhart (2004) states that infection with E. vermicularis causes symptoms of appendicitis (26). Hamood reported a case of acute appendicitis caused by E. vermicularis in a 23-year-old homemaker from Iraq in 2019. E. vermicularis can colonize the appendix and cause symptoms with or without actual histopathological acute appendicitis (28). The belief that E. vermicularis infestation can cause diseases such as acute or chronic appendicitis or perforation of the appendix has been documented (29). However, there are also reports of completely asymptomatic patients (30). All these cases prove that there is a connection between the inflammation of the appendix and even ALL, which is also due to the weakening of the immune system in these cases. Based on the results of previous studies and the importance of this parasite due to its effects on growth, health, nutritional status, and cognitive development (Especially in children), it is proposed that in order to control and prevent the disease, families should be given the necessary information about the life cycle, transmission, and prevention of infection with enterobiasis. In addition, since the infection caused by this parasite is easily transmissible, training should be provided to individuals, and personal and public health care should be improved to avoid the risk of infection with the parasite. Basic measures should also be taken to improve the economic situation of people. It is also recommended that public and private health promotion be considered through education.
Conclusion
According to the results of this report and other studies on this subject, it has been established that infection with this nematode may be associated with inflammation of the appendix or ALL. This is also true in cases where the immune system is weakened (e.g., in AIDS or congenital immunodeficiency syndromes) or when taking immunosuppressants (Special drugs for organ transplants). Therefore, a pinworm screening program remains necessary for immunocompromised patients or those receiving immunosuppressive medications.
Acknowledgement
The authors thank Dr. Davood Badbarin for his valuable suggestions and all the colleagues at the Children’s Hospital of Tabriz.
Funding sources
This study did not receive any funding.
Ethical statement
Since this manuscript is an unusual case report that does not violate the principles of ethics, ethical certification was not required. Therefore, the information's results were published in accordance with the principle of confidentiality.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
Case report: AR and SHGH; data collection: FA, MKH, and AR; data analysis: MKH; draft manuscript: MKH; intellectual contribution and manuscript revision: MKH. All authors read the manuscript and approved its final version.
Data availability statement
All data in this study is publicly available.
Research Article: Case Report |
Subject:
Parasitology
Received: 2023/07/1 | Accepted: 2023/10/9 | Published: 2025/02/19 | ePublished: 2025/02/19
Received: 2023/07/1 | Accepted: 2023/10/9 | Published: 2025/02/19 | ePublished: 2025/02/19
References
1. American Academy of Pediatrics. Pinworm infection (Enterobius vermicularis) Elk Grove Village, Illinois: American Academy of Pediatrics; 2006,520–522. [View at Publisher] [DOI] [Google Scholar]
2. Shoup B. Diagnosis and management of pinworm infection. Primary Care Update for OB/GYNS. 2001;8(6):240-3. [View at Publisher] [DOI] [Google Scholar]
3. Lee SE, Lee JH, Ju JW, Lee WJ, Cho SH. Prevalence of Enterobius vermicularis among preschool children in Gimhae-si, Gyeongsangnam-do, Korea. Korean J Parasitol. 2011;49(2):183-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Lala S, Upadhyay V. Enterobius vermicularis and its role in paediatric appendicitis: protection or predisposition. ANZ J Surg. 2016;86(9):717-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Boas H, Tapia G, Sodahl JA, Rasmussen T, Ronningen KS. Enterobius vermicularis and risk factors in healthy Norwegian children. Pediatr Infect Dis J. 2012;31(9):927-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Sodergren MH, Jethwa P, Wilkinson S, Kerwat R. Presenting features of Enterobius vermicularis in the vermiform appendix. Scand J Gastroenterol. 2009;44(4):457-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. da Silva DF, da Silva RJ, da Silva MG, Sartorelli AC, Rodrigues MA. Parasitic infection of the appendix as a cause of acute appendicitis. Parasitol Res. 2007;102(1):99-102. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Ariyarathenam AV, Nachimuthu S, Tang TY, Courtney ED, Harris SA, Harris AM. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int J Surg. 2010;8(6):466-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Wendt S, Trawinski H, Schubert S, Rodloff AC, Mössner J, Lübbert C. The Diagnosis and Treatment of Pinworm Infection. Dtsch Arztebl Int. 2019; 116(13):213-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Caldwell JP. Pinworms (enterobius vermicularis). Canadian Family Physician. 1982;28:306-9. [View at Publisher] [Google Scholar]
11. Budd JS, Armstrong C. Role of Enterobius vermicularis in the aetiology of appendicitis. Br J Surg. 1987;74(8):748-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Khubchandani IT, Bub DS. Parasitic Infections. Clin Colon Rectal Surg. 2019;32(5):364-71. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Purohit G, Mohanty S, Tirkey R, Sasmal PK. Inadvertent detection of massive Enterobius vermicularis infection in an asymptomatic adult with rectal blowout following barotrauma. Ann Parasitol. 2019;65(1):103-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Arca MJ, Gates RL, Groner JI, Hammond S, Caniano DA. Clinical manifestations of appendiceal pinworms in children: an institutional experience and a review of the literature. Pediatr Surg Int. 2004;20(5):372-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Gialamas E, Papavramidis T, Michalopoulos N, Karayannopoulou G, Cheva A, Vasilaki O, et al. Enterobius vermicularis: a rare cause of appendicitis. Turkiye Parazitol Derg. 2012;36(1):37-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Ramezani MA, Dehghani MR. Relationship between Enterobius vermicularis and the incidence of acute appendicitis. Southeast Asian J Trop Med Public Health. 2007;38(1):20-3. [View at Publisher] [PMID] [Google Scholar]
17. Gatti S, Lopes R, Cevini C, Ijaoba B, Bruno A, Bernuzzi AM, et al. Intestinal parasitic infections in an institution for the mentally retarded. Ann Trop Med Parasitol. 2000;94(5):453-60. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Yildirim S, Nursal TZ, Tarim A, Kayaselcuk F, Noyan T. A rare cause of acute appendicitis: parasitic infection. Scand J Infect Dis. 2005;37(10):757-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Mardani A, Feizi F, Fakhar M, Farrokhi M, Abbasi M, Asfaram S. Enterobius vermicularis infection among appendectomy specimens in Qom Province, Central Iran: a retrospective study. Comparative Clinical Pathology. 2017;26(5):1213-9. [View at Publisher] [DOI] [Google Scholar]
20. Dahlstrom JE, Macarthur EB. Enterobius vermicularis: a possible cause of symptoms resembling appendicitis. Aust N Z J Surg. 1994;64(10):692-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Dalimi A, Khoshzaban F. Comparative study of two methods for the diagnosis of Enterobius vermicularis in the appendix. J Helminthol. 1993;67(1):85-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Siavoshi M, Sattari M, Pile- Var M. Histopathology and Relative Frequency of Parasitic Appendicitis in the Main Treatment Centers of Hamadan City. Journal title. 1999;8(29 and 30):63-8. [View at Publisher] [Google Scholar]
23. Isik B, Yilmaz M, Karadag N, Kahraman L, Sogutlu G, Yilmaz S, et al. Appendiceal Enterobius vermicularis infestation in adults. Int Surg. 2007; 92(4): 221-225. [View at Publisher] [PMID] [Google Scholar]
24. Aydin O. Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diagn Pathol. 2007;2:16. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Gutierrez Y, Diagnostic Pathology of Parasitic Infections with Clinical Correlations. Oxford University Press;2000. p.521-3. [View at Publisher] [DOI] [Google Scholar]
26. Burkhart CN, Burkhart CG. Assessment of frequency, transmission, and genitourinary complications of enterobiasis (pinworms). Int J Dermatol. 2005;44(10):837-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Cook G. Enterobius vermicularis infection. Gut. 1994;35(9):1159. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Hammood ZD, Salih AM, Mohammed SH, Kakamad FH, Salih KM, Omar DA, et al. Enterobius vermicularis causing acute appendicitis, a case report with literature review. Int J Surg Case Rep. 2019;63:153-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Madhukar KP, Verma R, Sayed Z, Vira L. Acute appendicitis secondary to enterobius vermicularis infestation in a young female: a case report. J. Evol. Med. Dent. Sci. 2014;3(19):5219-23. [View at Publisher]
30. Gialamas E, Papavramidis T, Michalopoulos N, et al. Enterobius vermicularis: a rare cause of appendicitis. Turkiye Parazitol Derg. 2012;36(1):37-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com