Volume 17, Issue 6 (Nov-Dec 2023)
mljgoums 2023, 17(6): 1-3 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Devara M S, Bhamidipati S, Dondapati V B, Bandaru N R. Antibacterial activity of cinnamon extract against gram-positive and gram-negative bacterial
pathogens isolated from patient samples. mljgoums 2023; 17 (6) :1-3
URL: http://mlj.goums.ac.ir/article-1-1671-en.html
URL: http://mlj.goums.ac.ir/article-1-1671-en.html
Manasa Sireesha Devara1 

 , Sriushaswini Bhamidipati2
, Sriushaswini Bhamidipati2 

 , Vijaya Bharathi Dondapati3
, Vijaya Bharathi Dondapati3 

 , Narasinga Rao Bandaru4
, Narasinga Rao Bandaru4 




 , Sriushaswini Bhamidipati2
, Sriushaswini Bhamidipati2 

 , Vijaya Bharathi Dondapati3
, Vijaya Bharathi Dondapati3 

 , Narasinga Rao Bandaru4
, Narasinga Rao Bandaru4 


1- Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Visakhapatnam, India , devaramanasa18@gmail.com
2- Department of Surgery, Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Gvpihcmt, Visakhapatnam, India
3- Department of Microbiology, Gayatri Vidya Parishad Institute of Health care and Medical Technology, Gvpihcmt, Visakhapatnam, India
4- Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Visakhapatnam, India
2- Department of Surgery, Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Gvpihcmt, Visakhapatnam, India
3- Department of Microbiology, Gayatri Vidya Parishad Institute of Health care and Medical Technology, Gvpihcmt, Visakhapatnam, India
4- Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Visakhapatnam, India
Full-Text [PDF 385 kb]
(1384 Downloads)
| Abstract (HTML) (2781 Views)
Full-Text: (1439 Views)
Introduction
In the present days, multidrug-resistant microbial infections have become a major threat to patients who have taken several drugs for different infections. Misuse of antimicrobial drugs, illiteracy, and low production of newer antimicrobial drugs might spread multidrug-resistant strains in the population. The recent upsurge of antimicrobial drug resistance and its spread has posed a unique challenge to the global Infectious Diseases Control Programme (1-4). A total of 122 compounds were identified; 80% of these compounds were used for the same (or related) ethnomedical purposes (traditional medicine). Further, it was discovered that these compounds were derived from only 94 species of plants (5). Medicinal plants are rich in a wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, flavonoids, phenols, and quinones, which have been used worldwide in traditional medicine to treat several diseases and infections (6). Cinnamon (Cinnamomum zeylanicum) has been used in food preparations and in traditional medicine by the Egyptians and the Chinese since ancient times. Approximately 250 species of cinnamon genus have been identified globally. They are used as a flavoring agent and have some beneficial effects in medicinal, antimicrobial, and antioxidant applications. Cinnamon also acts as a natural food preservative. The medicinal applications of cinnamon include the treatment of diarrhea, flatulent dyspepsia, influenza, cough, bronchitis, angina, palpitations, controlling infections, and reducing blood sugar in diabetics. Cinnamon exhibits anti-inflammatory, antimycotic, insecticidal, and anticancer properties (7). Phytochemical components of photobiotics have been shown to exhibit high antibacterial action against Gram-positive and Gram-negative bacteria in both in vivo and in vitro environments. Chemical compounds are found in various parts of the cinnamon tree, where leaves contain 70-95% eugenol and 1-5% cinnamaldehyde, and the bark contains 5-10% eugenol and 65-80% cinnamaldehyde (8). A study showed that cinnamaldehyde exhibits its antimicrobial activity due to its lipophilicity of terpenoids and phenylpropanoids, which can penetrate the membrane, reach the inner part of the cell, and impair the bacterial enzyme system, which kills the micro-organism (9). The present study aimed to identify the antibacterial activity of cinnamon bark extract against human pathogenic bacterial isolates from clinical pus samples, which may help develop novel drugs to prevent multidrug-resistant emerging strains.
Methods
The present study was approved by the Institutional Ethics Committee (Ref. No. GVPIHCMT/IEC/20210204/02), and the study was carried out for 1 month in March 2023 at the Department of Microbiology, Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Visakhapatnam, Andhra Pradesh, India. Six pathogenic bacterial isolates were randomly taken from the routine cultures obtained from patient pus samples of the Surgical Ward, and the antibacterial potential of cinnamaldehyde and eugenol of the cinnamon bark extract diluted in both methanol and ethanol (Cinnamomum zeylanicum) was checked by agar well diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC).
Cinnamon extracts were prepared in accordance with the Ethiopian traditional way of processing these products for usage. Briefly, cinnamon barks (Figure 1a) were cleaned with deionized water and dried in the sunlight for 2 days. The barks were further dried in an oven (at 40 °C) for 30 minutes and ground into fine powders (Figure 1b). Cinnamon extract was prepared by soaking 10 g of the powder in 50 mL of absolute ethanol and methanol in a 250-mL Erlenmeyer flask. The flask was sealed with aluminum foil and placed on a shaker for 48 hr. at room temperature. Following the incubation, the extract was centrifuged at 3 500 RPM for 20 min and filtered through Whitman filter paper No. 1. The filtrate was dried at 40 °C in a dry oven until a semi-solid substance was obtained and was then further dried in a crucible at 45 °C. The extract was stored at -20 ºC until use. Methanol and ethanol extracts were dissolved in dimethyl sulfoxide (DMSO) to enhance permeability (1).

A total of 6 pathogenic bacterial strains were used to screen the antimicrobial activity of the cinnamon extracts. Bacterial isolates were identified based on colony morphology, Gram stain, motility, and biochemical reactions using standard techniques (10). Six pathogenic bacterial isolates (Staphylococcus aureus, coagulase-negative Staphylococci, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa) were isolated from patient pus samples received in the Microbiology Laboratory.
Bacterial inocula were prepared by subculturing a loopful of each strain in nutrient agar plates at 37 °C for 24 hr. Colonies from the overnight growth were picked with a sterile loop and inoculated into the sterile test tubes with 3 mL of the nutrient broth. The turbidity of the bacterial suspension was adjusted to 0.5 McFarland’s standard, giving a bacterial count of about 1 × 108 CFU/ mL (11).
The antibacterial activity of the cinnamon extract was tested against 6 strains of pathogenic bacteria using the agar-well diffusion assay. Briefly, 100 μL of each bacterial suspension, equivalent to 0.5 McFarland’s standard, was evenly distributed on the Mueller-Hinton agar (MHA). Then, 50 μL of the extract was applied to the wells prepared using a sterile 4-mm glass well borer. Ciprofloxacin disc and dimethyl sulfoxide (DMSO) were included for the positive and negative controls, respectively. The plates were then incubated at 37 °C for 24 hr. After the incubation period, the antimicrobial activity of each sample was determined by measuring the diameter of the inhibition zone around the wells (11).
The MIC was determined using the broth microdilution, as described previously (1,12). The cinnamon extracts were serially diluted to concentrations ranging from 3.125 mg/mL to 100 mg/mL. Then, 100 μL of test organisms, 5 × 105 CFU, was added to duplicate wells of each dilution and incubated at 37 °C for 24 hr. The DMSO-treated wells and wells with no bacterium (media only) were included as controls. The lowest concentration at which the bacteria did not show visible growth was defined as the MIC of a test sample. The minimum bactericidal concentration (MBC) was determined by inoculating 100 μL of the culture medium from the wells with no visible growth in the MIC test and subculturing on a fresh MHA at 37 °C for 24 hr. The concentration at which no visible bacterial colonies were seen on MHA was considered the MBC of the test sample (13).
Results
In the present study, the highest inhibitory activity of ethanol and methanol extracts of cinnamon was shown by S. aureus (20.36 mm and 19.44 mm), followed by coagulase-negative Staphylococci (14.33 mm and 18.01 mm), E. coli (17.58 mm and 16.47 mm), P. aeruginosa (17.56 mm and 16.33 mm), and K. pneumoniae (15.32 mm and 14.59 mm). The lowest inhibitory effect was shown by P. mirabilis (13.67 mm and 12.59 mm) by the agar dilution method (Figure 2). In the present study, the highest zone of inhibition for ciprofloxacin disc (5 µg) was 21.33 mm, which was used as the positive control. There is no zone of inhibition for DMSO, which was used as the negative control. The ethanol extract of cinnamon MIC and MBC against the test isolate ranged from 6.25 mg/mL to 12.5 mg/mL and from 12.5 mg/mL to 50 mg/mL. The methanol extract of cinnamon MIC showed a value of 12.5 mg/mL, and the methanol extract of MBC ranged from 12.5 mg/mL to 50 mg/mL against all clinical isolates of the present study (Table 1).

Discussion
In the present study, ethanol and methanol extracts of cinnamon bark powder showed antibacterial activity against 6 bacterial isolates obtained from clinical pus samples. The highest zone of inhibition was seen with S. aureus (20.36 mm and 19.44mm) with both the methanol and ethanol extracts of cinnamon and also approximate to the ciprofloxacin disc zone of inhibition (21.33 mm), which was comparable with Hassan A. Hussein et al. (14) and Asha, S et al. (15). Nishteman F. Mohammad (16) reported that S. aureus confers resistance to all concentrations of cinnamon extract. On observation, the highest zone of inhibition with ethanol extract showed action on more bacteria than the methanol extract, which was comparable to Sriushaswini et al. (17) and Asha S et al. (15). In the present study, the MIC and MBC of S. aureus and coagulase-negative Staphylococcus showed the lowest concentration for both ethanol (6.25 mg/mL and 12.5 mg/mL) and methanol (12.5 mg/mL and 25 mg/mL) extract of cinnamon when compared to other MIC and MBC of bacterial isolates (Table 1), whereas Deressa T et al. (1) reported that MIC and MBC of cinnamon ethanol and methanol extract for S. aureus showed 12.5 and 25 mg/mL and 6.25 and 25 mg/mL; MIC and MBC of cinnamon ethanol and methanol extracts for coagulase-negative Staphylococcus showed 50 mg/mL and 25 mg/mL and 50mg/mL, respectively. In clinical reports, it was found to be very safe and useful in allergic conditions (18). Since ancient times, a variety of plants and their components have been in use for their medicinal properties (19). Some of these have also been identified for their antibacterial activity against resistant pathogens (20,21). Emerging resistance of microorganisms to conventional chemicals and drugs has prompted scientists to search for novel sources of biocides with broad-spectrum activities (22,23).
Conclusion
In the present study, the S. aureus isolate showed the highest inhibition of growth in both ethanol and methanol extracts of cinnamon bark. The ethanol solvent of cinnamon bark extract showed better antimicrobial potential than the methanol solvent. We are further conducting in vitro studies, which are necessary to know the antimicrobial activity of different organisms other than what we included in the present study.
Acknowledgement
We acknowledge the help of the faculty and technical staff of the Microbiology Department.
Funding sources
This study did not receive any grant from funding agencies in the public, commercial, or non-profit organizations.
Ethical statement
Institutional Ethics Committee – Ref. No. GVPIHCMT/IEC/20210204/02.
Conflicts of interest
There are no conflicts of interest to declare by any of the authors.
Author contributions
1) Manasa Sireesha, MD. received the pus samples, performed the agar well diffusion, minimum inhibitory concentration, and minimum bactericidal concentration for the antibacterial activity of cinnamon, and drafted the manuscript.
2) Sriushaswini, MS, helped to send clinical pus samples from the Surgical
Department.
3) B. Narasinga Rao, MD, PhD, checked the prepared manuscript.
4) Vijaya Bharathi, MD, re-checked the manuscript.
In the present days, multidrug-resistant microbial infections have become a major threat to patients who have taken several drugs for different infections. Misuse of antimicrobial drugs, illiteracy, and low production of newer antimicrobial drugs might spread multidrug-resistant strains in the population. The recent upsurge of antimicrobial drug resistance and its spread has posed a unique challenge to the global Infectious Diseases Control Programme (1-4). A total of 122 compounds were identified; 80% of these compounds were used for the same (or related) ethnomedical purposes (traditional medicine). Further, it was discovered that these compounds were derived from only 94 species of plants (5). Medicinal plants are rich in a wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, flavonoids, phenols, and quinones, which have been used worldwide in traditional medicine to treat several diseases and infections (6). Cinnamon (Cinnamomum zeylanicum) has been used in food preparations and in traditional medicine by the Egyptians and the Chinese since ancient times. Approximately 250 species of cinnamon genus have been identified globally. They are used as a flavoring agent and have some beneficial effects in medicinal, antimicrobial, and antioxidant applications. Cinnamon also acts as a natural food preservative. The medicinal applications of cinnamon include the treatment of diarrhea, flatulent dyspepsia, influenza, cough, bronchitis, angina, palpitations, controlling infections, and reducing blood sugar in diabetics. Cinnamon exhibits anti-inflammatory, antimycotic, insecticidal, and anticancer properties (7). Phytochemical components of photobiotics have been shown to exhibit high antibacterial action against Gram-positive and Gram-negative bacteria in both in vivo and in vitro environments. Chemical compounds are found in various parts of the cinnamon tree, where leaves contain 70-95% eugenol and 1-5% cinnamaldehyde, and the bark contains 5-10% eugenol and 65-80% cinnamaldehyde (8). A study showed that cinnamaldehyde exhibits its antimicrobial activity due to its lipophilicity of terpenoids and phenylpropanoids, which can penetrate the membrane, reach the inner part of the cell, and impair the bacterial enzyme system, which kills the micro-organism (9). The present study aimed to identify the antibacterial activity of cinnamon bark extract against human pathogenic bacterial isolates from clinical pus samples, which may help develop novel drugs to prevent multidrug-resistant emerging strains.
Methods
The present study was approved by the Institutional Ethics Committee (Ref. No. GVPIHCMT/IEC/20210204/02), and the study was carried out for 1 month in March 2023 at the Department of Microbiology, Gayatri Vidya Parishad Institute of Health Care and Medical Technology, Visakhapatnam, Andhra Pradesh, India. Six pathogenic bacterial isolates were randomly taken from the routine cultures obtained from patient pus samples of the Surgical Ward, and the antibacterial potential of cinnamaldehyde and eugenol of the cinnamon bark extract diluted in both methanol and ethanol (Cinnamomum zeylanicum) was checked by agar well diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC).
Cinnamon extracts were prepared in accordance with the Ethiopian traditional way of processing these products for usage. Briefly, cinnamon barks (Figure 1a) were cleaned with deionized water and dried in the sunlight for 2 days. The barks were further dried in an oven (at 40 °C) for 30 minutes and ground into fine powders (Figure 1b). Cinnamon extract was prepared by soaking 10 g of the powder in 50 mL of absolute ethanol and methanol in a 250-mL Erlenmeyer flask. The flask was sealed with aluminum foil and placed on a shaker for 48 hr. at room temperature. Following the incubation, the extract was centrifuged at 3 500 RPM for 20 min and filtered through Whitman filter paper No. 1. The filtrate was dried at 40 °C in a dry oven until a semi-solid substance was obtained and was then further dried in a crucible at 45 °C. The extract was stored at -20 ºC until use. Methanol and ethanol extracts were dissolved in dimethyl sulfoxide (DMSO) to enhance permeability (1).

A total of 6 pathogenic bacterial strains were used to screen the antimicrobial activity of the cinnamon extracts. Bacterial isolates were identified based on colony morphology, Gram stain, motility, and biochemical reactions using standard techniques (10). Six pathogenic bacterial isolates (Staphylococcus aureus, coagulase-negative Staphylococci, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa) were isolated from patient pus samples received in the Microbiology Laboratory.
Bacterial inocula were prepared by subculturing a loopful of each strain in nutrient agar plates at 37 °C for 24 hr. Colonies from the overnight growth were picked with a sterile loop and inoculated into the sterile test tubes with 3 mL of the nutrient broth. The turbidity of the bacterial suspension was adjusted to 0.5 McFarland’s standard, giving a bacterial count of about 1 × 108 CFU/ mL (11).
The antibacterial activity of the cinnamon extract was tested against 6 strains of pathogenic bacteria using the agar-well diffusion assay. Briefly, 100 μL of each bacterial suspension, equivalent to 0.5 McFarland’s standard, was evenly distributed on the Mueller-Hinton agar (MHA). Then, 50 μL of the extract was applied to the wells prepared using a sterile 4-mm glass well borer. Ciprofloxacin disc and dimethyl sulfoxide (DMSO) were included for the positive and negative controls, respectively. The plates were then incubated at 37 °C for 24 hr. After the incubation period, the antimicrobial activity of each sample was determined by measuring the diameter of the inhibition zone around the wells (11).
The MIC was determined using the broth microdilution, as described previously (1,12). The cinnamon extracts were serially diluted to concentrations ranging from 3.125 mg/mL to 100 mg/mL. Then, 100 μL of test organisms, 5 × 105 CFU, was added to duplicate wells of each dilution and incubated at 37 °C for 24 hr. The DMSO-treated wells and wells with no bacterium (media only) were included as controls. The lowest concentration at which the bacteria did not show visible growth was defined as the MIC of a test sample. The minimum bactericidal concentration (MBC) was determined by inoculating 100 μL of the culture medium from the wells with no visible growth in the MIC test and subculturing on a fresh MHA at 37 °C for 24 hr. The concentration at which no visible bacterial colonies were seen on MHA was considered the MBC of the test sample (13).
Results
In the present study, the highest inhibitory activity of ethanol and methanol extracts of cinnamon was shown by S. aureus (20.36 mm and 19.44 mm), followed by coagulase-negative Staphylococci (14.33 mm and 18.01 mm), E. coli (17.58 mm and 16.47 mm), P. aeruginosa (17.56 mm and 16.33 mm), and K. pneumoniae (15.32 mm and 14.59 mm). The lowest inhibitory effect was shown by P. mirabilis (13.67 mm and 12.59 mm) by the agar dilution method (Figure 2). In the present study, the highest zone of inhibition for ciprofloxacin disc (5 µg) was 21.33 mm, which was used as the positive control. There is no zone of inhibition for DMSO, which was used as the negative control. The ethanol extract of cinnamon MIC and MBC against the test isolate ranged from 6.25 mg/mL to 12.5 mg/mL and from 12.5 mg/mL to 50 mg/mL. The methanol extract of cinnamon MIC showed a value of 12.5 mg/mL, and the methanol extract of MBC ranged from 12.5 mg/mL to 50 mg/mL against all clinical isolates of the present study (Table 1).

Table 1. Antibacterial activity of cinnamon bark extract by MIC and MBC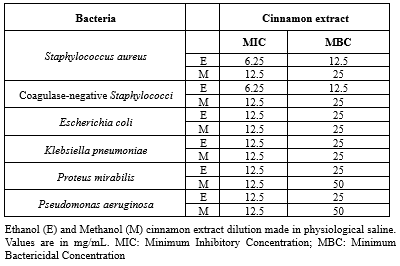 |
Discussion
In the present study, ethanol and methanol extracts of cinnamon bark powder showed antibacterial activity against 6 bacterial isolates obtained from clinical pus samples. The highest zone of inhibition was seen with S. aureus (20.36 mm and 19.44mm) with both the methanol and ethanol extracts of cinnamon and also approximate to the ciprofloxacin disc zone of inhibition (21.33 mm), which was comparable with Hassan A. Hussein et al. (14) and Asha, S et al. (15). Nishteman F. Mohammad (16) reported that S. aureus confers resistance to all concentrations of cinnamon extract. On observation, the highest zone of inhibition with ethanol extract showed action on more bacteria than the methanol extract, which was comparable to Sriushaswini et al. (17) and Asha S et al. (15). In the present study, the MIC and MBC of S. aureus and coagulase-negative Staphylococcus showed the lowest concentration for both ethanol (6.25 mg/mL and 12.5 mg/mL) and methanol (12.5 mg/mL and 25 mg/mL) extract of cinnamon when compared to other MIC and MBC of bacterial isolates (Table 1), whereas Deressa T et al. (1) reported that MIC and MBC of cinnamon ethanol and methanol extract for S. aureus showed 12.5 and 25 mg/mL and 6.25 and 25 mg/mL; MIC and MBC of cinnamon ethanol and methanol extracts for coagulase-negative Staphylococcus showed 50 mg/mL and 25 mg/mL and 50mg/mL, respectively. In clinical reports, it was found to be very safe and useful in allergic conditions (18). Since ancient times, a variety of plants and their components have been in use for their medicinal properties (19). Some of these have also been identified for their antibacterial activity against resistant pathogens (20,21). Emerging resistance of microorganisms to conventional chemicals and drugs has prompted scientists to search for novel sources of biocides with broad-spectrum activities (22,23).
Conclusion
In the present study, the S. aureus isolate showed the highest inhibition of growth in both ethanol and methanol extracts of cinnamon bark. The ethanol solvent of cinnamon bark extract showed better antimicrobial potential than the methanol solvent. We are further conducting in vitro studies, which are necessary to know the antimicrobial activity of different organisms other than what we included in the present study.
Acknowledgement
We acknowledge the help of the faculty and technical staff of the Microbiology Department.
Funding sources
This study did not receive any grant from funding agencies in the public, commercial, or non-profit organizations.
Ethical statement
Institutional Ethics Committee – Ref. No. GVPIHCMT/IEC/20210204/02.
Conflicts of interest
There are no conflicts of interest to declare by any of the authors.
Author contributions
1) Manasa Sireesha, MD. received the pus samples, performed the agar well diffusion, minimum inhibitory concentration, and minimum bactericidal concentration for the antibacterial activity of cinnamon, and drafted the manuscript.
2) Sriushaswini, MS, helped to send clinical pus samples from the Surgical
Department.
3) B. Narasinga Rao, MD, PhD, checked the prepared manuscript.
4) Vijaya Bharathi, MD, re-checked the manuscript.
Research Article: Research Article |
Subject:
bacteriology
Received: 2023/05/29 | Accepted: 2023/11/26 | Published: 2024/02/26 | ePublished: 2024/02/26
Received: 2023/05/29 | Accepted: 2023/11/26 | Published: 2024/02/26 | ePublished: 2024/02/26
References
1. Deressa T, Tamiru T, Biadgo B, Belete D, Zewdu S, Mengistie S, et al. Antimicrobial Potentials of Apis Multiflora Honey in Combination with Coffee and Cinnamon Extracts against Common Human Pathogenic Bacteria. Med Aromat Plants. 2015;4(4):1-5. [View at Publisher] [DOI] [Google Scholar]
2. Rossolini GM, D'Andrea MM, Mugnaioli C The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008;14(S1):33-41. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Patel RV, Thaker VT, Patel VK. Anitmicrobial activity of ginger and honey on isolates of extracted carious teeth during orthodontic treatment. Asian Pac J Trop Biomed. 2011;1(S1): S58-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. New Delhimetallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg Infect Dis. 2011;17(1):103-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Fabricant DS, Farnsworth NR. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ Health Perspect. 2001;109(S1):69-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Abdulrasheed M, Ibrahim IH, Luka A, Maryam AA, Hafsat L, Ibrahim S1, et al. Antibacterial Effect of Cinnamon (Cinnamomum zeylanicum) Bark Extract on Different Bacterial Isolates. J Environ Microbiol and Toxicology. 2019;7(1):16-20. [View at Publisher] [DOI] [Google Scholar]
7. Adarsh A, Bharath Chettiyar, Kanthesh BM, Raghu N. Phytochemical Screening and Antimicrobial Activity of "Cinnamon zeylanicum" International Journal of Pharmaceutical Research and Innovation. 2020;13:22-33. [View at Publisher] [Google Scholar]
8. Patel KM, Parmar BB, Sadariya KA, Bhavsar SK. Assessment of in vitro antibacterial activity and MIC of cinnamon bark powder ethanolic and aqueous extracts against bacteria. J Phytopharm. 2022;11(5):324-9. [View at Publisher] [DOI] [Google Scholar]
9. Rajesh P, Rajesh Kannan V, Latha S, Selvamani P. Phytochemical and pharmacological profile of plants belonging to Strychnos genus; a review. Bioact Phytochem Perspect Mod Med. 2014;1:275-327. [View at Publisher] [Google Scholar]
10. Mackie TJ, Collee JG, McCartney JE, editors. Mackie and McCartney Practical Medical Microbiology. A Guide to the Laboratory Diagnosis and Control of infection.13th ed. V2. New York: Churchill Livingstone.1989. [View at Publisher] [Google Scholar]
11. Andrews JM, BASC Working Party on Susceptibility Testing. BSAC standard disc susceptibility testing method 12. (version 5). J Antimicrob Chemother. 2006;58(3):511-29. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Shahidi Bonjar GH. Evaluation of antimicrobial properties of Iranian medicinal plants against Micrococcus luteus, Serratia marceceans, Klebsiella pneumoniae and Bordetella bronchoseptica. Asian J Plant Sci. 2004;3(1):82-6. [View at Publisher] [DOI] [Google Scholar]
13. Julianti E, Rajah KK, Fidrianny I. Antibacterial Activity Ethanolic Extract of Cinnamon Bark,Honey and their combination effects against AcneCausing Bacteria. Sci Pharm. 2017;85(2):19. [View at Publisher] [DOI] [Google Scholar]
14. Hussein HA, Abbas IS, Hussain Ali R. Antibacterial activities of Cinnamon zeylanicum Syzygium aromaticum Essential oil. Int J Pharm Pharm Sci. 2014;6(5):165-8. [View at Publisher] [Google Scholar]
15. Asha S, Nithisha k, Bharath Kumar R, Ravi kumar V. Deciphering the Antimicrobial Potential of Cinnamon zeylanicum Bark. Int J Pharmatech Rec. 2014;6(4):1226-35. [View at Publisher] [Google Scholar]
16. Nishteman FM, Shilan SS, Rand BB, Dian JS. Antibacterial activies of plant extract Cinnamomum zeylanicum bark against multidrug-resistant bacteria. Irq J Pharm. 2021;18(2):10-21. [View at Publisher] [DOI] [Google Scholar]
17. Sriushaswini B, Vidyasagar KVSB, Voleti A, Perala Balamurali Krishna and Bandaru Narasinga Rao. Antibacterial activity of Asafoetida against Human Pathogenic Bacteria Obtained from Surgical Units of a Tertiary Care Hospital. J Hosp Pharmacy. 2021;16(2):1-8. [View at Publisher] [Google Scholar]
18. Jakhetia V, Patel R, Khatri P, Pahuja N, Garg S, Pandey A, et al. Cinnamon: a pharmacological-review. Journal of Advanced Scientific Research. 2010;1(2):19-2. [View at Publisher] [Google Scholar]
19. Meckes M, Villarreal ML, Tortoriello J, Berlin B, Berlin EA. A microbiological evaluation of medicinal plants used by the Maya people of Southern Mexico. Phytother Res. 1995;9(4):244-50. [View at Publisher] [DOI] [Google Scholar]
20. Sagun E, Durmaz H, Tarakci Z, Sagdic O. Antibacterial activities of extracts of some herbs used in Turkish herby cheese against Listeria monocytogenes serovars. Int J Food Proper. 2006;9(2):255-60. [View at Publisher] [DOI] [Google Scholar]
21. Dondapati VB, Bandaru S, Babu SK, Bandaru NR, Perala BK, Voleti A, et al. Antibacterial activity of coffee extract against common human bacterial pathogens in a teaching hospital of semi urban setup. Natl J of Physiol Pharm Pharmacol. 2023;13(4):773-7. [View at Publisher] [DOI] [Google Scholar]
22. Jose Abad M, Ansuategui M, Bermejo P. Active antifungal substances from natural sources. ARKIVOC. 2007;Vii:116-45. [View at Publisher] [DOI] [Google Scholar]
23. Oulkheir S, Aghrouch M, El Mourabit F, Dalha F, Graich H, Amouch F, et al. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium Against a Gram negative and Gram positive Pathogenic Bacteria. Journal of Diseases and Medicinal Plants. 2017;3(2):1-5. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





