Volume 18, Issue 3 (May-Jun 2024)
mljgoums 2024, 18(3): 25-28 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shafieian R, Ebrahimzadeh-Bideskan A, Ranjbar E. Evaluation of different decalcifying agents on histochemical and immunohistochemical staining properties of canine osseous tissue. mljgoums 2024; 18 (3) :25-28
URL: http://mlj.goums.ac.ir/article-1-1599-en.html
URL: http://mlj.goums.ac.ir/article-1-1599-en.html
1- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
3- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran ,ranjbar.esmaeil.mums@gmail.com
2- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
3- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran ,
Full-Text [PDF 604 kb]
(1373 Downloads)
| Abstract (HTML) (4319 Views)
Full-Text: (1839 Views)
Introduction
Decalcification is an essential step routinely performed for histopathological observation of bone and bone-containing tissues. The purpose of decalcification is to remove calcium salts from mineralized tissue in such a way that the essential microscopic components are properly preserved for sectioning through routine histopathological methods (1). Calcification can be used to examine oral pathology (2) and bone metastases (3). Numerous methods have been employed for decalcification, including heat, microwave, electric current, and chemical agents. Among these methods, chemical agents are the most common tool in routine histopathological analysis (4,5). The most extensively employed chemical agents for decalcification are either acids, which form soluble calcium salts, or chelating agents such as ethylene diamine tetraacetic acid (EDTA), which function by binding to calcium ions on the surface of apatite crystals (6). Each method has its own advantages and drawbacks. Faster, acidic solutions may adversely affect the tissues, resulting in minimal to severe damage to the staining characteristics of the organic components. On the other hand, chelation preserves the organic phase of tissues sufficiently intact but is a slow process and not ideal for routine use (7). Thus, an ideal decalcifying approach should preserve tissue morphology and antigenicity within a reasonable time limit. Selecting the appropriate decalcification protocol involves balancing the speed of decalcification with the preservation of tissue morphology and staining quality.
According to the authors' literature search, the effects of decalcifying reagents and practical details of decalcification for canine osseous tissue are largely unknown. Thus, we presented a comparative evaluation of four different decalcifying agents for canine mandibular tissue, focusing on the decalcification time, ease of tissue slicing, the agents’ impact on histological staining characteristics, and antigenic preservation.
Methods
All animals were housed and euthanized following the National Institutes of Health guidelines for the care and use of laboratory animals. Four healthy male Mongrel dogs (2 ± 0.1 years old) were housed in individual cages, provided with controlled temperature (20 ± 2 °C), lighting (12 hr light: 12 hr dark photoperiod), and free access to water ad libitum, as well as commercially balanced dry food (France). After two weeks of adaptation, a total of 32 samples of mandibular bone tissue (Eight from each animal) were harvested under general anesthesia, which was induced and maintained under the supervision of a veterinarian. All surgical procedures were performed under aseptic conditions.
Fixation of the harvested bone tissue was accomplished using 10% neutral-buffered formalin (ThermoFisher Scientific, Waltham, MA) for 72 hr followed by overnight rinsing in running water for the next 24 hr. Decalcification of the samples was performed using either 5% nitric acid (5% NA), 10% formic acid (10% FA), 20% formic acid (20% FA), or 10% EDTA (pH 7.4) at room temperature (Table 1). For decalcification, the tissue samples were completely immersed in approximately 100 ml of the solution, with the solution volume being 10 times the tissue volume, and received repeated agitation each day. In addition, decalcifying solutions were changed every other day, except for 10% EDTA, which was replaced weekly. The total time required for complete decalcification was recorded precisely. To achieve randomization, each decalcifying solution contained eight fixed samples (Two from each animal) to account for variability between the animals.
The endpoint of the decalcification process was determined using the following methods (6):
After determining the endpoint time of decalcification, the specimens were washed in PBS, followed by routine dehydration and paraffin embedding processes, as described elsewhere (8). The prepared blocks were then stored at room temperature for further use.
The efficiency of decalcifying agents was assessed at four levels during the investigation: first, the speed of decalcification was evaluated according to the endpoint criteria; second, ease of sectioning was assessed during the microtome cutting process; third, morphological preservation was evaluated with Alizarin red S (AZR) staining; and fourth, antigenic preservation of osteocalcin (OCN) was assessed via immunohistochemistry (IHC) staining.
Sections of 6 µm thickness were acquired with a microtome (HM 325 Microm). Afterward, tissue slides underwent AZR staining, as described earlier (9).
Morphological preservation of the bone tissue was evaluated based on the following criteria:
IHC processing was carried out on specimens placed on adhesive-coated glass slides (3-aminopropyltriethoxysilane, Sigma-Aldrich) to prevent detachment. The primary rabbit antibody against osteocalcin (ab93876, Abcam, USA) was used, as described earlier (8). All slides were independently evaluated and graded under an optical microscope (Olympus, Japan) by two independent observers. The slides were graded based on the intensity of immunoreactions using a four-tier grading system: 4 (Strong), 3 (Moderate), 2 (Mild), and 1 (None). Initially, the slides were evaluated to determine the range of immunoreactivity for each type of decalcifying solution, with the strongest reactivity being graded as 4.
Statistical analysis was performed using the SPSS 11.5 software package for Windows. The efficiency of the decalcifying solutions was analyzed statistically using one-way ANOVA. The Kruskal-Wallis test was used to determine if there were significant differences between the solutions tested for each parameter evaluated across the four experiments. Differences with P < 0.05 were considered significant.
Results
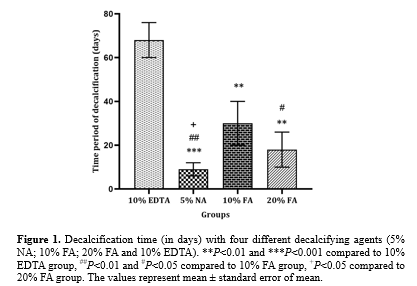
The mean decalcification time for each experimental group was recorded as follows: 8.1 days for 5% NA, 30.4 days for 10% FA, 17.8 days for 20% FA, and 67.6 days for 10% EDTA. The mean decalcification time was significantly shorter with 5% NA compared to 10% EDTA (P < 0.001), 10% FA (P < 0.01), and 20% FA (P < 0.05). In addition, the decalcification time for 20% FA was significantly shorter than for 10% EDTA (P<0.01) and 10% FA (P < 0.05). The mean decalcification time for 10% FA was also significantly shorter than for 10% EDTA (P < 0.01) (Figure. 1).
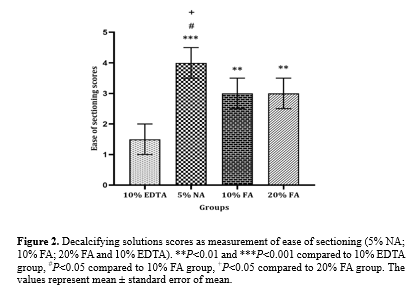
After paraffin embedding, tissues decalcified with 5% NA showed greater ease in microtome cutting compared to those treated with 10% EDTA (P<0.001), 10% FA, and 20% FA (P < 0.05). Tissues treated with 10% FA and 20% FA showed tolerable ease in sectioning, which was significantly higher than that for 10% EDTA (P < 0.01). The hardest specimens to cut were those decalcified with 10% EDTA (Figure. 2).
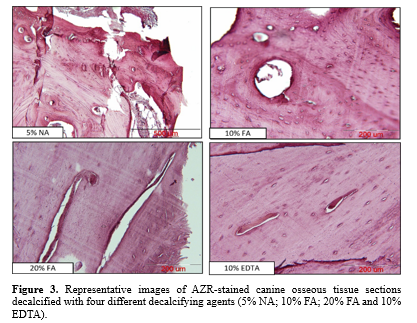
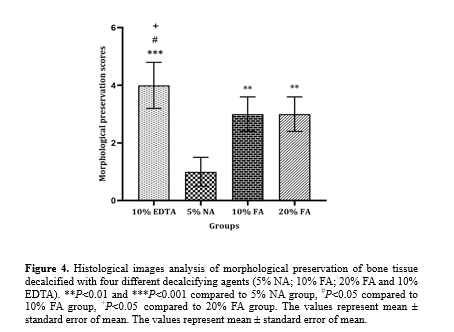
AZR staining (Figure. 3) indicated that decalcification with 10% EDTA provided the best preservation of cellular structures compared to 10% and 20% FA (P < 0.05) and 5% NA (P < 0.001). No significant difference was observed in the preservation of cellular structures between tissues decalcified with 10% and 20% FA. In contrast, tissue morphology and the uniformity of soft tissue were mostly affected in tissue sections decalcified with 5% NA, which was significantly lower than in tissue sections decalcified with 10% and 20% FA (P < 0.01) (Figure. 4).
Antigenic preservation of OCN in decalcified tissue sections was evaluated by observing positive immunoreacted cells with brown cytoplasm, indicating the accumulation of OCN protein (Figure. 5).
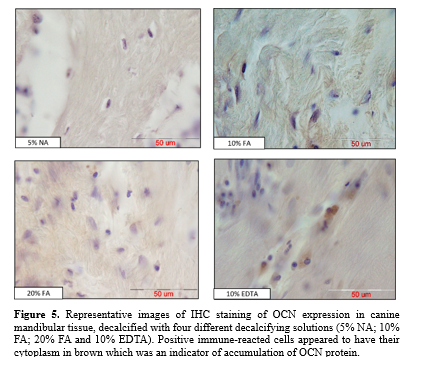
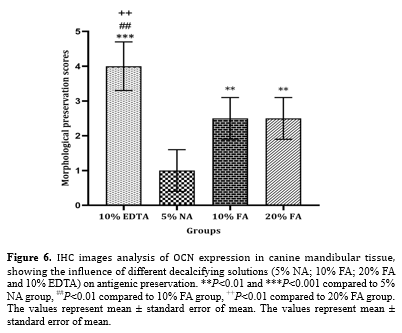
Stronger immunoreactivity was observed during decalcification with 10% EDTA compared to 10% and 20% FA (P < 0.01) and 5% NA (P < 0.001). Moderate immunoreactivity was noted during decalcification with 10% and 20% FA, with no significant difference between the two. Decalcification with 5% NA showed significantly lower immunoreactivity compared to 10% and 20% FA (P < 0.01) and almost failed to preserve antigenicity (Figure. 6).
Discussion
In the present study, the effects of four different decalcifying solutions on the decalcification time, ease of sectioning, and quality of AZR and IHC staining were compared using canine mandibular bone tissue. Regarding the decalcification time and ease of sectioning, 5% NA yielded better results than 10% and 20% FA and 10% EDTA (pH 7.4). For the preservation of morphology and antigenicity of the tissue samples, 10% EDTA (pH 7.4) was found to be the most optimal solution, followed by 10% and 20% FA and 5% NA.
Previous studies comparing different decalcifying solutions with respect to factors such as time period and morphological preservation reported diverse and sometimes, controversial results, which can be confusing in laboratory practices (10,11). The type and concentration of the decalcifying solution are crucial variables that significantly impact the decalcification time, ease of sectioning, and morphological and antigenic preservation throughout the bone tissue. However, regardless of the decalcifying agent, these factors also depend largely on the type of tissue being decalcified. Therefore, this issue should also be considered.
EDTA is one of the most commonly used decalcifying agents in histopathological laboratories due to its satisfactory antigenic preservation, as shown in this study. However, the advantage of EDTA is often overshadowed by its time-consuming incubation process (12). The decalcification time reported for 10% EDTA varies widely, depending on the sample size and host (13). Liu et al. reported a 21-day decalcification period for rat femurs (13). However, another study described a 100-day lag for the decalcification of rat hind paws (1). This variation may result from differences in specimen size and the presence of skin surrounding the specimen. In this study, decalcification of canine alveolar bone using 10% EDTA took approximately 67 days, making it the most laborious compared to the other solutions.
Efficacious IHC requires selecting a decalcifying solution that preserves the results. Several studies have examined the immunoreactivity of various molecules after using either acid or chelating demineralizing solutions (14). For example, an assessment of different decalcifying protocols on Osteopontin (OPN) and OCN immunostaining in bone specimens from an arthritis rat model using confocal immunofluorescence showed that the optimal solution for detecting OPN and OCN was 5% NA, followed by 10% EDTA (pH 7.4) (1). Our results also indicated that the highest quality in preserving the antigenicity and morphology of canine mandibular tissue was achieved with 10% EDTA (pH 7.4). The superior results may be attributed to the chelating agent's mechanism of capturing metallic ions, such as calcium, layer-by-layer from the outer layer of the apatite crystals to the deeper layers. In this way, the crystal size decreases gradually and steadily, leading to an excellent preservation of tissue components (11).
However, immunostaining details and morphological preservation were adversely affected with 5% NA, according to our findings. These variations might be due to differences in the type of tissue and host. On the other hand, 5% NA significantly reduced the decalcification time to the minimum level and provided the highest ease during tissue sectioning.
Formic acid is well-accepted and widely used for decalcification, likely due to its time-saving nature and ability to preserve morphology, as noted in various studies (15,16). Bogoevski et al. reported that FA is a good alternative to EDTA, although EDTA remains the best option for tissue preservation quality (17). In this study, the decalcification time significantly increased from 5% NA to 10% EDTA, while 10% and 20% FA showed intermediate results, with a significant decrease in time from 10% FA to 20% FA. Indeed, a 100% increase in the concentration of FA resulted in a significant reduction of about 39% in the decalcification time. This effect may be attributed to the increased speed of penetration throughout the samples. Despite the rapid action of 5% NA, FA operates more slowly, which helps in better preservation of bone tissue structure (13).
Regardless of the solution used, decalcification methods are generally accelerated by supplementary factors such as increased controlled temperature (17). This study focused on different decalcifying agents and did not employ such factors, thereby standardizing the procedure. Other limitations include the use of only one decalcifying agent per experiment, which may have restricted the potential for novel findings. Moreover, using different fixatives prior to decalcification could yield altered results. To date, no original research has compared mineralizing solutions specifically for decalcifying canine osseous tissue, making this study the first to address this issue.
Conclusion
According to the results of this study, decalcification with 10% EDTA (pH 7.4) was preferable due to its maintenance of the integrity and antigenic reactivity of canine mandibular tissue, making it the best choice for cases where time is not a critical factor. Conversely, 20% FA demonstrated a balance between tissue integrity and decalcification time, suggesting its suitability as a stable decalcifying agent for routine histopathological diagnoses. In contrast, 5% NA may be considered when only general staining protocols are required.
Acknowledgement
The authors thank Mrs. Fatemeh Motejadded for her technical support.
Funding sources
Funding was provided by the Vice Chancellor for Research of Mashhad University of Medical Sciences (Grant number: 971876).
Ethical statement
The study was performed in accordance with the National Institutes of Health guidelines outlined in the "Guide for the Care and Use of Laboratory Animals," published by the National Academies Press.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
All authors contributed to data collection, data analysis, and writing of the manuscript.
Decalcification is an essential step routinely performed for histopathological observation of bone and bone-containing tissues. The purpose of decalcification is to remove calcium salts from mineralized tissue in such a way that the essential microscopic components are properly preserved for sectioning through routine histopathological methods (1). Calcification can be used to examine oral pathology (2) and bone metastases (3). Numerous methods have been employed for decalcification, including heat, microwave, electric current, and chemical agents. Among these methods, chemical agents are the most common tool in routine histopathological analysis (4,5). The most extensively employed chemical agents for decalcification are either acids, which form soluble calcium salts, or chelating agents such as ethylene diamine tetraacetic acid (EDTA), which function by binding to calcium ions on the surface of apatite crystals (6). Each method has its own advantages and drawbacks. Faster, acidic solutions may adversely affect the tissues, resulting in minimal to severe damage to the staining characteristics of the organic components. On the other hand, chelation preserves the organic phase of tissues sufficiently intact but is a slow process and not ideal for routine use (7). Thus, an ideal decalcifying approach should preserve tissue morphology and antigenicity within a reasonable time limit. Selecting the appropriate decalcification protocol involves balancing the speed of decalcification with the preservation of tissue morphology and staining quality.
According to the authors' literature search, the effects of decalcifying reagents and practical details of decalcification for canine osseous tissue are largely unknown. Thus, we presented a comparative evaluation of four different decalcifying agents for canine mandibular tissue, focusing on the decalcification time, ease of tissue slicing, the agents’ impact on histological staining characteristics, and antigenic preservation.
Methods
All animals were housed and euthanized following the National Institutes of Health guidelines for the care and use of laboratory animals. Four healthy male Mongrel dogs (2 ± 0.1 years old) were housed in individual cages, provided with controlled temperature (20 ± 2 °C), lighting (12 hr light: 12 hr dark photoperiod), and free access to water ad libitum, as well as commercially balanced dry food (France). After two weeks of adaptation, a total of 32 samples of mandibular bone tissue (Eight from each animal) were harvested under general anesthesia, which was induced and maintained under the supervision of a veterinarian. All surgical procedures were performed under aseptic conditions.
Fixation of the harvested bone tissue was accomplished using 10% neutral-buffered formalin (ThermoFisher Scientific, Waltham, MA) for 72 hr followed by overnight rinsing in running water for the next 24 hr. Decalcification of the samples was performed using either 5% nitric acid (5% NA), 10% formic acid (10% FA), 20% formic acid (20% FA), or 10% EDTA (pH 7.4) at room temperature (Table 1). For decalcification, the tissue samples were completely immersed in approximately 100 ml of the solution, with the solution volume being 10 times the tissue volume, and received repeated agitation each day. In addition, decalcifying solutions were changed every other day, except for 10% EDTA, which was replaced weekly. The total time required for complete decalcification was recorded precisely. To achieve randomization, each decalcifying solution contained eight fixed samples (Two from each animal) to account for variability between the animals.
|
Table 1. The constituents and preparation method of decalcifying solutions
 |
- Physical Method: Bending and needling of the bone tissue using a surgical blade (Without applying force)
- Chemical Evaluation: Calcium-oxalate test, which detects insoluble calcium oxalate salt precipitated as small white spots in the solution
- Radiographic Assessment: Evaluating bone samples for relative opacity to suggest complete decalcification
After determining the endpoint time of decalcification, the specimens were washed in PBS, followed by routine dehydration and paraffin embedding processes, as described elsewhere (8). The prepared blocks were then stored at room temperature for further use.
The efficiency of decalcifying agents was assessed at four levels during the investigation: first, the speed of decalcification was evaluated according to the endpoint criteria; second, ease of sectioning was assessed during the microtome cutting process; third, morphological preservation was evaluated with Alizarin red S (AZR) staining; and fourth, antigenic preservation of osteocalcin (OCN) was assessed via immunohistochemistry (IHC) staining.
Sections of 6 µm thickness were acquired with a microtome (HM 325 Microm). Afterward, tissue slides underwent AZR staining, as described earlier (9).
Morphological preservation of the bone tissue was evaluated based on the following criteria:
- Preservation of bone organization, including bone trabeculae, bone marrow spaces, and osteocytes within their lacunae
- Preservation of the osteoblastic cell layer
- High preservation of staining characteristics
IHC processing was carried out on specimens placed on adhesive-coated glass slides (3-aminopropyltriethoxysilane, Sigma-Aldrich) to prevent detachment. The primary rabbit antibody against osteocalcin (ab93876, Abcam, USA) was used, as described earlier (8). All slides were independently evaluated and graded under an optical microscope (Olympus, Japan) by two independent observers. The slides were graded based on the intensity of immunoreactions using a four-tier grading system: 4 (Strong), 3 (Moderate), 2 (Mild), and 1 (None). Initially, the slides were evaluated to determine the range of immunoreactivity for each type of decalcifying solution, with the strongest reactivity being graded as 4.
Statistical analysis was performed using the SPSS 11.5 software package for Windows. The efficiency of the decalcifying solutions was analyzed statistically using one-way ANOVA. The Kruskal-Wallis test was used to determine if there were significant differences between the solutions tested for each parameter evaluated across the four experiments. Differences with P < 0.05 were considered significant.
Results
The mean decalcification time for each experimental group was recorded as follows: 8.1 days for 5% NA, 30.4 days for 10% FA, 17.8 days for 20% FA, and 67.6 days for 10% EDTA. The mean decalcification time was significantly shorter with 5% NA compared to 10% EDTA (P < 0.001), 10% FA (P < 0.01), and 20% FA (P < 0.05). In addition, the decalcification time for 20% FA was significantly shorter than for 10% EDTA (P<0.01) and 10% FA (P < 0.05). The mean decalcification time for 10% FA was also significantly shorter than for 10% EDTA (P < 0.01) (Figure. 1).

After paraffin embedding, tissues decalcified with 5% NA showed greater ease in microtome cutting compared to those treated with 10% EDTA (P<0.001), 10% FA, and 20% FA (P < 0.05). Tissues treated with 10% FA and 20% FA showed tolerable ease in sectioning, which was significantly higher than that for 10% EDTA (P < 0.01). The hardest specimens to cut were those decalcified with 10% EDTA (Figure. 2).

AZR staining (Figure. 3) indicated that decalcification with 10% EDTA provided the best preservation of cellular structures compared to 10% and 20% FA (P < 0.05) and 5% NA (P < 0.001). No significant difference was observed in the preservation of cellular structures between tissues decalcified with 10% and 20% FA. In contrast, tissue morphology and the uniformity of soft tissue were mostly affected in tissue sections decalcified with 5% NA, which was significantly lower than in tissue sections decalcified with 10% and 20% FA (P < 0.01) (Figure. 4).


Antigenic preservation of OCN in decalcified tissue sections was evaluated by observing positive immunoreacted cells with brown cytoplasm, indicating the accumulation of OCN protein (Figure. 5).

Stronger immunoreactivity was observed during decalcification with 10% EDTA compared to 10% and 20% FA (P < 0.01) and 5% NA (P < 0.001). Moderate immunoreactivity was noted during decalcification with 10% and 20% FA, with no significant difference between the two. Decalcification with 5% NA showed significantly lower immunoreactivity compared to 10% and 20% FA (P < 0.01) and almost failed to preserve antigenicity (Figure. 6).

Discussion
In the present study, the effects of four different decalcifying solutions on the decalcification time, ease of sectioning, and quality of AZR and IHC staining were compared using canine mandibular bone tissue. Regarding the decalcification time and ease of sectioning, 5% NA yielded better results than 10% and 20% FA and 10% EDTA (pH 7.4). For the preservation of morphology and antigenicity of the tissue samples, 10% EDTA (pH 7.4) was found to be the most optimal solution, followed by 10% and 20% FA and 5% NA.
Previous studies comparing different decalcifying solutions with respect to factors such as time period and morphological preservation reported diverse and sometimes, controversial results, which can be confusing in laboratory practices (10,11). The type and concentration of the decalcifying solution are crucial variables that significantly impact the decalcification time, ease of sectioning, and morphological and antigenic preservation throughout the bone tissue. However, regardless of the decalcifying agent, these factors also depend largely on the type of tissue being decalcified. Therefore, this issue should also be considered.
EDTA is one of the most commonly used decalcifying agents in histopathological laboratories due to its satisfactory antigenic preservation, as shown in this study. However, the advantage of EDTA is often overshadowed by its time-consuming incubation process (12). The decalcification time reported for 10% EDTA varies widely, depending on the sample size and host (13). Liu et al. reported a 21-day decalcification period for rat femurs (13). However, another study described a 100-day lag for the decalcification of rat hind paws (1). This variation may result from differences in specimen size and the presence of skin surrounding the specimen. In this study, decalcification of canine alveolar bone using 10% EDTA took approximately 67 days, making it the most laborious compared to the other solutions.
Efficacious IHC requires selecting a decalcifying solution that preserves the results. Several studies have examined the immunoreactivity of various molecules after using either acid or chelating demineralizing solutions (14). For example, an assessment of different decalcifying protocols on Osteopontin (OPN) and OCN immunostaining in bone specimens from an arthritis rat model using confocal immunofluorescence showed that the optimal solution for detecting OPN and OCN was 5% NA, followed by 10% EDTA (pH 7.4) (1). Our results also indicated that the highest quality in preserving the antigenicity and morphology of canine mandibular tissue was achieved with 10% EDTA (pH 7.4). The superior results may be attributed to the chelating agent's mechanism of capturing metallic ions, such as calcium, layer-by-layer from the outer layer of the apatite crystals to the deeper layers. In this way, the crystal size decreases gradually and steadily, leading to an excellent preservation of tissue components (11).
However, immunostaining details and morphological preservation were adversely affected with 5% NA, according to our findings. These variations might be due to differences in the type of tissue and host. On the other hand, 5% NA significantly reduced the decalcification time to the minimum level and provided the highest ease during tissue sectioning.
Formic acid is well-accepted and widely used for decalcification, likely due to its time-saving nature and ability to preserve morphology, as noted in various studies (15,16). Bogoevski et al. reported that FA is a good alternative to EDTA, although EDTA remains the best option for tissue preservation quality (17). In this study, the decalcification time significantly increased from 5% NA to 10% EDTA, while 10% and 20% FA showed intermediate results, with a significant decrease in time from 10% FA to 20% FA. Indeed, a 100% increase in the concentration of FA resulted in a significant reduction of about 39% in the decalcification time. This effect may be attributed to the increased speed of penetration throughout the samples. Despite the rapid action of 5% NA, FA operates more slowly, which helps in better preservation of bone tissue structure (13).
Regardless of the solution used, decalcification methods are generally accelerated by supplementary factors such as increased controlled temperature (17). This study focused on different decalcifying agents and did not employ such factors, thereby standardizing the procedure. Other limitations include the use of only one decalcifying agent per experiment, which may have restricted the potential for novel findings. Moreover, using different fixatives prior to decalcification could yield altered results. To date, no original research has compared mineralizing solutions specifically for decalcifying canine osseous tissue, making this study the first to address this issue.
Conclusion
According to the results of this study, decalcification with 10% EDTA (pH 7.4) was preferable due to its maintenance of the integrity and antigenic reactivity of canine mandibular tissue, making it the best choice for cases where time is not a critical factor. Conversely, 20% FA demonstrated a balance between tissue integrity and decalcification time, suggesting its suitability as a stable decalcifying agent for routine histopathological diagnoses. In contrast, 5% NA may be considered when only general staining protocols are required.
Acknowledgement
The authors thank Mrs. Fatemeh Motejadded for her technical support.
Funding sources
Funding was provided by the Vice Chancellor for Research of Mashhad University of Medical Sciences (Grant number: 971876).
Ethical statement
The study was performed in accordance with the National Institutes of Health guidelines outlined in the "Guide for the Care and Use of Laboratory Animals," published by the National Academies Press.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
All authors contributed to data collection, data analysis, and writing of the manuscript.
Research Article: Research Article |
Subject:
Pathology
Received: 2022/11/30 | Accepted: 2023/04/8 | Published: 2024/06/8 | ePublished: 2024/06/8
Received: 2022/11/30 | Accepted: 2023/04/8 | Published: 2024/06/8 | ePublished: 2024/06/8
References
1. González-Chávez SA, Pacheco-Tena C, Macías-Vázquez CE, Luévano-Flores E. Assessment of different decalcifying protocols on Osteopontin and Osteocalcin immunostaining in whole bone specimens of arthritis rat model by confocal immunofluorescence. Int J Clin Exp Pathol. 2013; 6(10): 1972-83. [View at Publisher] [PMID] [Google Scholar]
2. Bhaskar SN. Orban's oral histology and embriology. Orban's oral histology and embriology. 2012; 482. [View at Publisher]
3. Singh VM, Salunga RC, Huang VJ, Tran Y, Erlander M, Plumlee P, et al. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Annals of Diagnostic Pathology. 2013 ;17(4): 322-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Savi FM, Brierly GI, Baldwin J, Theodoropoulos C, Woodruff MA. Comparison of different decalcification methods using rat mandibles as a model. Journal of Histochemistry & Cytochemistry. 2017; 65(12): 705-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Chow DH, Zheng L, Tian L, Ho K-S, Qin L, Guo X. Application of ultrasound accelerates the decalcification process of bone matrix without affecting histological and immunohistochemical analysis. Journal of orthopaedic translation. 2019; 17: 112-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Gupta S, Jawanda MK, Manjunath S, Bharti A. Qualitative histological evaluation of hard and soft tissue components of human permanent teeth using various decalcifying agents-a comparative study. Journal of clinical and diagnostic research: JCDR. 2014; 8(9): ZC69. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Kapila SN, Natarajan S, Boaz K, Pandya JA, Yinti SR. Driving the mineral out faster: simple modifications of the decalcification technique. Journal of clinical and diagnostic research: JCDR. 2015; 9(9): ZC93. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Shafieian R, Matin MM, Rahpeyma A, Fazel A, Sedigh HS, Sadr-Nabavi A, et al. The effect of platelet-rich plasma on human mesenchymal stem cell-induced bone regeneration of canine alveolar defects with calcium phosphate-based scaffolds. Iranian journal of basic medical sciences. 2017;20(10):1131. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Williams TW. Alizarin red S and toluidine blue for differentiating adult or embryonic bone and cartilage. Stain Technology. 1941; 16(1): 23-5. [View at Publisher] [DOI] [Google Scholar]
10. Emans P, Bulstra S, Kuijer R. The effects of different decalcification protocols on TUNEL and general cartilage staining. Biotechnic & Histochemistry. 2005;80(3-4):111-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Mattuella LG, Bento LW, Vier-Pelisser FV, Araujo FB, Fossati ACM. Comparative analysis of two fixating and two decalcifying solutions for processing of human primary teeth with inactive dentin carious lesion. Revista Odonto Ciência. 2007; 22(56): 99-105. [View at Publisher] [Google Scholar]
12. Choi S-E, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. Journal of pathology and translational medicine. 2015; 49(3): 236. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Liu H, Zhu R, Liu C, Ma R, Wang L, Chen B, et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. BioMed research international. 2017;2017; 9050754. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Miquelestorena-Standley E, Jourdan M-L, Collin C, Bouvier C, Larousserie F, Aubert S, et al. Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples. Modern Pathology. 2020; 33(8): 1505-1517. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Sangeetha R, Uma K, Chandavarkar V. Comparison of routine decalcification methods with microwave decalcification of bone and teeth. Journal of oral and maxillofacial pathology: JOMFP. 2013; 17(3): 386. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Sanjai K, Kumarswamy J, Patil A, Papaiah L, Jayaram S, Krishnan L. Evaluation and comparison of decalcification agents on the human teeth. Journal of oral and maxillofacial pathology: JOMFP. 2012; 16(2): 222. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Bogoevski K, Woloszyk A, Blackwood K, Woodruff MA, Glatt V. Tissue morphology and antigenicity in mouse and rat tibia: Comparing 12 different decalcification conditions. Journal of Histochemistry & Cytochemistry. 2019; 67(8): 545-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Senturk YECGE. Preparation Techniques of Luminal and Hard Tissues for Scanning Electron Microscopy.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com