Volume 17, Issue 4 (Jul-Aug 2023)
mljgoums 2023, 17(4): 5-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Metkar G, Saraf S. The value of coagulation parameters as prognostic indicators in snake envenomation. mljgoums 2023; 17 (4) :5-8
URL: http://mlj.goums.ac.ir/article-1-1577-en.html
URL: http://mlj.goums.ac.ir/article-1-1577-en.html
1- Latis CHS, Talegaon Chakan Road, Talegaon (D), Pune, Maharashtra, India
2- Akola, Maharashtra, India , drshalakasaraf@gmail.com
2- Akola, Maharashtra, India , drshalakasaraf@gmail.com
Full-Text [PDF 437 kb]
(279 Downloads)
| Abstract (HTML) (1050 Views)
Full-Text: (211 Views)
Introduction
Snakebite is a potentially life-threatening emergency that can be treated effectively (1-6). Snake envenomation is a major health problem in India (7-10). India is an agrarian country, and most of the population of India dwells in rural areas. The rural farming population most often faces the occupational hazard of snake envenomation (11-15) due to the intersection of the breeding season of prey of snakes and flooding of the natural habitat of snakes with the farming season of humans (16-21). Snake envenomation can cause hematologic abnormalities, which can present as bleeding manifestations, capillary leak syndrome, or disseminated intravascular coagulation (22-24), among which venom-induced consumption coagulopathy (VICC) is commonest (17-22). Previously, VICC was misinterpreted as disseminated intravascular coagulation. Similar to VICC, this was due to a deranged coagulation profile in DIC in the form of elevated D-dimer, prolonged prothrombin time (PT), and low fibrinogen levels. However, it should be noted that VICC differs from disseminated intravascular coagulation with respect to the absence of systemic microthrombi, absence of end-organ failure, rapid onset and resolution, difference in pathogenesis, and lower mortality compared to DIC (22). For timely detection of coagulation abnormalities and VICC, coagulation tests, such as PT, international normalized ratio (INR), and activated partial thromboplastin time (aPTT), are of utmost importance. Coagulation tests also help in monitoring patient treatment (25-30). The present study aimed to determine the value of coagulation parameters in the prognostication of snakebite patients.
Methods
All consecutive cases of snake envenomation of all age groups and genders admitted in a tertiary health care hospital from October 2019 to August 2021 were included in this prospective descriptive observational study. Patients with a history of administration of anti-snake venom (ASV) before reaching the study setting, patients with pre-existing coagulopathy (ascertained from clinical history and previous investigations), patients already on anticoagulants and antiplatelet drugs, and pregnant females with snakebite were excluded from the study. Coagulation parameters (such as PT, INR, and aPTT) were studied in all patients on admission and, if found deranged, were repeated at intervals of 12 hours till they returned to normal levels. Prothrombin time assesses coagulation factors in the extrinsic and common pathways. These coagulation factors include factors VII, X, and V, prothrombin, and fibrinogen. Samples for coagulation tests were collected in sodium citrate vacutainers. The PT-INR test was performed on a STAGO semi-automated coagulation analyzer using NeoPTimal plus reagent, which contains thromboplastin and calcium chloride. The blood samples of healthy controls were also taken and similarly processed. The values of PT were observed and classified as normal (less than 17 seconds) and abnormal (more than 17 seconds) (30). Also, INR was calculated with the help of the international sensitivity index (ISI) value of the reagent and internal control of the laboratory. The normal range for INR is less than 1.2. INR = (PT of patient/PT of control) ISI. On the basis of INR, patients were divided into 4 groups. Patients with INR more than 1.2 were divided into 3 groups as follows: 1.2- 4.0 - mildly deranged INR, 4.0-12.0 moderately deranged INR, and 12.0-20.0 severe deranged INR (17-22). Activated partial thromboplastin time (aPTT) is a measure of function of coagulation factors of intrinsic and common pathways. These pathways are controlled by coagulation factors XII and XI, high molecular weight kininogen, prekallikrein, factor IX, factor VIII, factor X, factor II, prothrombin, and fibrinogen. Thus, the activated partial thromboplastin time (aPTT) test measures overall efficiency of the intrinsic pathway and common pathway (31). aPTT was performed on a STAGO semi-automated coagulation analyzer using a reagent containing kaolin, phospholipid, and calcium chloride. In the laboratory, samples were centrifuged at 3000 rpm for 5 minutes to separate the plasma from the citrated blood sample. A volume of 0.1 mL of platelet-poor citrated plasma was collected, placed in a micro cuvette, kept in a well with a magnetic steel ball, and incubated for 1 minute. Then, the micro cuvette was placed in the coagulation well, and 0.2 mL of reagent was added to it. The coagulation analyzer detects the time of clot formation in seconds. The normal range for aPTT is 25-30 seconds. aPTT values were categorized into normal (less than or equal to 30 seconds) and abnormal (more than 30 seconds) groups (17-22). All the patients were followed up during their hospital stay, and the data of the total number of ASV vials administered and fresh frozen plasma (FFP) units administered, as well as the total duration of hospitalization and the number of days spent in the intensive care unit (ICU), were recorded.
All procedures performed in the current study were approved by the Institutional Review Board of MIMER Medical College, Talegaon Dabhade, India (code: MUHS/Medical/MUHS-020660/2019) in accordance with the 1964 Helsinki Declaration and its later amendments.
Informed consent was obtained from all participants included in the study.
Coagulation parameters on admission were correlated with each other using the Pearson correlation in SPSS version 26 (SPSS Inc, Chicago, IL, USA). P values less than 0.05 were considered statistically significant. The prognostic parameters used were the total number of ASV vials administered, the total number of days of hospital stay, the total number of days in ICU, the total number of FFP units administered, and the total number of blood components administered. Coagulation parameters (ie, PT, INR, and aPTT) were correlated with each of the prognostic parameters using the Pearson correlation in SPSS version 26. P values less than 0.05 were considered statistically significant. The international normalized ratio on admission and after 12 hours was correlated with the total number of days in the ICU using repeated measures analysis of variance (ANOVA) using SPSS software version 26. P values less than 0.05 were considered statistically significant.
Results
A total of 58 patients who met the inclusion criteria were included in the study between November 2019 and August 2021. Among these 58 patients, the majority, specifically 40 individuals (69%), were male. The highest proportion of patients (approximately 60%) belonged to the age group of 16-40 years. The age range of the participants varied from 6 to 72 years old.PT-INR was performed in all 58 patients on admission. If deranged, it was repeated after 12 hours. It was found that the number of times PT-INR was repeated was directly proportional to the severity of envenomation of that patient (Figure 1).

Prothrombin time on admission was graded into normal (≤17 seconds) and deranged (>17 seconds). Of the 58 patients, PT on admission was deranged (>17 seconds) in 81% of patients.
The international normalized ratio on admission was graded into normal (0-1.2), mildly (1.21-4.0), moderately (4.1-12.0), and severely deranged (12.1-20.0). Of the 58 patients, 26 (45%) had mildly deranged INR on admission, 3 (5%) had moderately deranged INR on admission, and 13 (22%) had severely deranged INR on admission.
PT-INR was repeated when clinically indicated or when the previous value was deranged. PT-INR after 12 hours of admission was performed on a total of 35 patients. It was graded into normal (0-1.2) and mildly deranged (1.21-4.0) values. Of the 58 patients, PT-INR was repeated in 35 patients. Of the 35 (77%) patients with repeated PT-INR, 27 showed mildly deranged INR, and 8 (23%) showed INR restoring to normal after administration of a repeat dose of ASV. PT-INR was repeated as indicated after 24 hours of admission in 17 (29.31%) patients. They were categorized into normal and mildly deranged groups.
Eleven (65%) patients had mildly deranged INR, and 6 (35%) patients had restoration of INR back to normal after administration of repeat doses of ASV and blood products.
aPTT was performed in all 58 patients on admission. They were categorized into normal (< 30 seconds) and deranged (>30 seconds) groups. Of the 58 patients, 48 (83%) had deranged aPTT, and 11 (17%) had normal aPTT.
A statistically significant correlation was observed between PT and aPTT values on admission (P = 0.008). The values of INR and aPTT were correlated with each other on admission, which was statistically significant (P = 0.008).
A statistically significant correlation was found between the total number of ASV vials administered and PT, INR, and aPTT values on admission (P = 0.004; Table 1).
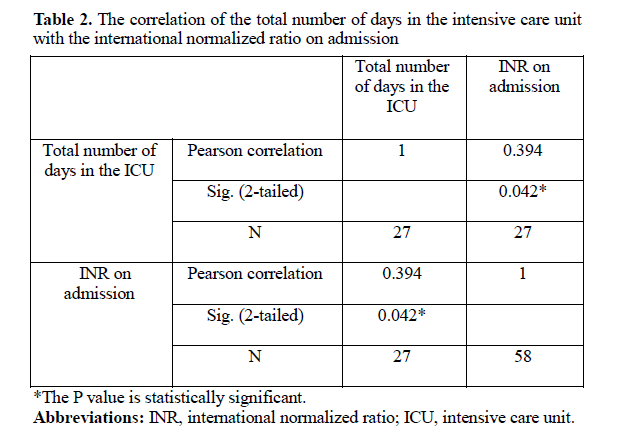
A significant correlation was found between the INR levels and the total number of days spent in the ICU on admission and after 12 hours of admission (P = 0.042; Table 2).

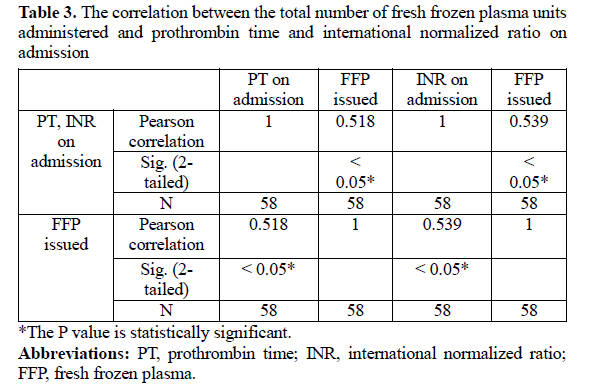
The correlation between other coagulation parameters was not statistically significant. A significant correlation was found between the total number of FFP units administered and PT and INR on admission (P < 0.05; Table 3). The correlation between the total number of FFP units administered and aPTT was not statistically significant (P = 0.832). The correlation between the total number of blood components administered and PT was statistically significant on admission (P = 0.001). The correlation between the total number of blood components administered and INR was statistically significant on admission (P ≤ 0.001). The correlation between the total number of blood components administered and aPTT was not statistically significant (P = 0.785).
Discussion
In our study, the majority of the patients were male (69%), which is consistent with the study by Agrawal et al (64%) and Hariprasad et al (75%) (17, 32). However, Karthikeyan et al showed a female predominance (51%) (30).
In our study, 81% of patients showed prolongation of PT on admission. Agrawal et al showed prolongation of PT-INR in 26% of cases, while Harshvardhana et al showed prolongation of PT (>15 seconds) in 56% of patients (32, 33). Karthikeyan et al showed prolongation of PT (PT > 17 seconds) in 75.6% of patients (30).
Ireland et al showed deranged INR (>1.2) in 86% of cases on admission (34). Prolongation of PT significantly correlated with the total number of ASV vials administered. Patients with deranged PT on admission required a greater number of ASV vials to achieve normal coagulation. Ramamurthy et al also showed the increased requirement of ASV with the prolongation of PT (33).
Prothrombin time >17 seconds was also associated with the increased requirement of FFP in the current study. Similarly, Harshvardhana et al showed that 136 FFP units were required for patients with PT of more than 15 seconds (33).
In the current study, PT-INR was performed on admission. Of the 58 patients, 26 (45%) had mildly deranged INR (1.2- 4.0), 3 (5%) had moderately deranged INR, and 13 (22%) had severely deranged INR on admission. Agrawal et al showed prolongation of INR in 26% of cases, while Hariprasad et al showed prolongation of INR (>1.2) in 85% of patients (17, 32). Ireland et al showed deranged INR (>1.2) in 86% of cases on admission (34).
In our study, it was observed that the administration of ASV resulted in the restoration of INR to normal levels in some cases. However, in a significant proportion of patients (specifically, 77% of those whose INR was reassessed), the INR remained prolonged even after 12 hours of admission. Prolongation of INR beyond 12 hours was seen in 3.7% of cases in the study by Agrawal et al (32). Ireland et al showed deranged INR beyond 12 hours in 14% of cases (34). Of the 17 patients, 11 (65%) required a repeat INR measurement 24 hours after admission, showing that the INR values were mildly deranged. However, in the existing literature, the prolongation of INR has only been studied for up to 12 hours. The international normalized ratio on admission and 12 hours after admission demonstrated a significant correlation with the total number of days spent in the ICU. Mean INR on admission and 12 hours after admission was 8.5 and 1.5, respectively, for 4 days in the ICU, whereas it was 1.4 and 1.3 for 1 day in the ICU. This result was significant as morbidity can be predicted significantly by following up INR 12 hours after admission. In our study, a significant correlation was found between the derangement of INR on admission and the total number of days spent in the ICU. Patients with severely deranged INR had a significantly longer duration of stay in the ICU compared with those with mildly deranged INR values. These results are consistent with the results of Hariprasad et al (17). In our study, a significant correlation was found between the derangement of INR on admission and the total number of ASV vials administered. This correlation was also significant in the studies by Ramamurthy et al and Harshvardhan et al (29, 35). Prolonged INR is thus an indication of a repeat dose of ASV and FFP.
In the current study, the total number of FFP units administered significantly correlated with INR on admission. Similarly, Harshvardhana et al found that 136 FFP units were required for 25 days of hospitalization days in patients with PT of more than 15 seconds. (33) In the study by Hariprasad et al, 112 patients with INR more than 1.5 also required FFP (17).
In our study, aPTT was prolonged in 83% of patients. Similar results were seen in the study by Patil et al (73.6%) and Agrawal et al (26.4%) (32, 35). In the current study, aPTT significantly correlated with the total number of ASV vials administered. Ramamurthy et al also showed that the prolongation of aPTT was associated with an increased requirement of ASV (29). In our study, prolonged aPTT was associated with increased days of hospitalization in the ICU. These results are consistent with the results of Harshvardhana et al (33).
Implications of the study
This study offers practical guidelines for the use of coagulation tests in the management of snakebite patients. It provides insights into the optimal timing and frequency of conducting coagulation tests in these patients, as well as predicting the need for ASV and blood products such as FFP.
Limitations of the study
In the present study, the proportion of mild to moderate cases was more than severe cases.
Conclusion
Coagulation parameters (ie, PT, INR, and aPTT) can be reliably used for predicting the requirement for ASV, FFP, other blood products, and duration of ICU stay. Thus, coagulation profile estimation can be used in predicting snakebite patients’ prognosis. To enhance the prognostic value of coagulation tests, it is recommended to repeat these tests at 12-hour intervals in patients with deranged coagulation profiles.
Acknowledgement
We acknowledge the Head of the Department of Pathology and MIMER Medical College for guidance and constant support.
Funding sources
No funding was used for this study.
Ethical statement
All procedures performed in the current study were approved by Institutional review board (IRB) of MIMER Medical College, Talegaon (D) (approval number- MUHS/Medical/MUHS-020660/2019) in accordance with the1964 Helsinki declaration and its later amendments.
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
The authors declare that they have no potential conflict of interest to disclose.
Author contributions
1. GSM contributed significantly in conceptualization, methodology, project administration, managing resources, supervising the project, validation visualization, writing original draft and writing review.
2. SPS has contributed significantly in data curation, formal analysis, conducting investigations, using software for statistical analysis and writing original draft.
Snakebite is a potentially life-threatening emergency that can be treated effectively (1-6). Snake envenomation is a major health problem in India (7-10). India is an agrarian country, and most of the population of India dwells in rural areas. The rural farming population most often faces the occupational hazard of snake envenomation (11-15) due to the intersection of the breeding season of prey of snakes and flooding of the natural habitat of snakes with the farming season of humans (16-21). Snake envenomation can cause hematologic abnormalities, which can present as bleeding manifestations, capillary leak syndrome, or disseminated intravascular coagulation (22-24), among which venom-induced consumption coagulopathy (VICC) is commonest (17-22). Previously, VICC was misinterpreted as disseminated intravascular coagulation. Similar to VICC, this was due to a deranged coagulation profile in DIC in the form of elevated D-dimer, prolonged prothrombin time (PT), and low fibrinogen levels. However, it should be noted that VICC differs from disseminated intravascular coagulation with respect to the absence of systemic microthrombi, absence of end-organ failure, rapid onset and resolution, difference in pathogenesis, and lower mortality compared to DIC (22). For timely detection of coagulation abnormalities and VICC, coagulation tests, such as PT, international normalized ratio (INR), and activated partial thromboplastin time (aPTT), are of utmost importance. Coagulation tests also help in monitoring patient treatment (25-30). The present study aimed to determine the value of coagulation parameters in the prognostication of snakebite patients.
Methods
All consecutive cases of snake envenomation of all age groups and genders admitted in a tertiary health care hospital from October 2019 to August 2021 were included in this prospective descriptive observational study. Patients with a history of administration of anti-snake venom (ASV) before reaching the study setting, patients with pre-existing coagulopathy (ascertained from clinical history and previous investigations), patients already on anticoagulants and antiplatelet drugs, and pregnant females with snakebite were excluded from the study. Coagulation parameters (such as PT, INR, and aPTT) were studied in all patients on admission and, if found deranged, were repeated at intervals of 12 hours till they returned to normal levels. Prothrombin time assesses coagulation factors in the extrinsic and common pathways. These coagulation factors include factors VII, X, and V, prothrombin, and fibrinogen. Samples for coagulation tests were collected in sodium citrate vacutainers. The PT-INR test was performed on a STAGO semi-automated coagulation analyzer using NeoPTimal plus reagent, which contains thromboplastin and calcium chloride. The blood samples of healthy controls were also taken and similarly processed. The values of PT were observed and classified as normal (less than 17 seconds) and abnormal (more than 17 seconds) (30). Also, INR was calculated with the help of the international sensitivity index (ISI) value of the reagent and internal control of the laboratory. The normal range for INR is less than 1.2. INR = (PT of patient/PT of control) ISI. On the basis of INR, patients were divided into 4 groups. Patients with INR more than 1.2 were divided into 3 groups as follows: 1.2- 4.0 - mildly deranged INR, 4.0-12.0 moderately deranged INR, and 12.0-20.0 severe deranged INR (17-22). Activated partial thromboplastin time (aPTT) is a measure of function of coagulation factors of intrinsic and common pathways. These pathways are controlled by coagulation factors XII and XI, high molecular weight kininogen, prekallikrein, factor IX, factor VIII, factor X, factor II, prothrombin, and fibrinogen. Thus, the activated partial thromboplastin time (aPTT) test measures overall efficiency of the intrinsic pathway and common pathway (31). aPTT was performed on a STAGO semi-automated coagulation analyzer using a reagent containing kaolin, phospholipid, and calcium chloride. In the laboratory, samples were centrifuged at 3000 rpm for 5 minutes to separate the plasma from the citrated blood sample. A volume of 0.1 mL of platelet-poor citrated plasma was collected, placed in a micro cuvette, kept in a well with a magnetic steel ball, and incubated for 1 minute. Then, the micro cuvette was placed in the coagulation well, and 0.2 mL of reagent was added to it. The coagulation analyzer detects the time of clot formation in seconds. The normal range for aPTT is 25-30 seconds. aPTT values were categorized into normal (less than or equal to 30 seconds) and abnormal (more than 30 seconds) groups (17-22). All the patients were followed up during their hospital stay, and the data of the total number of ASV vials administered and fresh frozen plasma (FFP) units administered, as well as the total duration of hospitalization and the number of days spent in the intensive care unit (ICU), were recorded.
All procedures performed in the current study were approved by the Institutional Review Board of MIMER Medical College, Talegaon Dabhade, India (code: MUHS/Medical/MUHS-020660/2019) in accordance with the 1964 Helsinki Declaration and its later amendments.
Informed consent was obtained from all participants included in the study.
Coagulation parameters on admission were correlated with each other using the Pearson correlation in SPSS version 26 (SPSS Inc, Chicago, IL, USA). P values less than 0.05 were considered statistically significant. The prognostic parameters used were the total number of ASV vials administered, the total number of days of hospital stay, the total number of days in ICU, the total number of FFP units administered, and the total number of blood components administered. Coagulation parameters (ie, PT, INR, and aPTT) were correlated with each of the prognostic parameters using the Pearson correlation in SPSS version 26. P values less than 0.05 were considered statistically significant. The international normalized ratio on admission and after 12 hours was correlated with the total number of days in the ICU using repeated measures analysis of variance (ANOVA) using SPSS software version 26. P values less than 0.05 were considered statistically significant.
Results
A total of 58 patients who met the inclusion criteria were included in the study between November 2019 and August 2021. Among these 58 patients, the majority, specifically 40 individuals (69%), were male. The highest proportion of patients (approximately 60%) belonged to the age group of 16-40 years. The age range of the participants varied from 6 to 72 years old.PT-INR was performed in all 58 patients on admission. If deranged, it was repeated after 12 hours. It was found that the number of times PT-INR was repeated was directly proportional to the severity of envenomation of that patient (Figure 1).

Prothrombin time on admission was graded into normal (≤17 seconds) and deranged (>17 seconds). Of the 58 patients, PT on admission was deranged (>17 seconds) in 81% of patients.
The international normalized ratio on admission was graded into normal (0-1.2), mildly (1.21-4.0), moderately (4.1-12.0), and severely deranged (12.1-20.0). Of the 58 patients, 26 (45%) had mildly deranged INR on admission, 3 (5%) had moderately deranged INR on admission, and 13 (22%) had severely deranged INR on admission.
PT-INR was repeated when clinically indicated or when the previous value was deranged. PT-INR after 12 hours of admission was performed on a total of 35 patients. It was graded into normal (0-1.2) and mildly deranged (1.21-4.0) values. Of the 58 patients, PT-INR was repeated in 35 patients. Of the 35 (77%) patients with repeated PT-INR, 27 showed mildly deranged INR, and 8 (23%) showed INR restoring to normal after administration of a repeat dose of ASV. PT-INR was repeated as indicated after 24 hours of admission in 17 (29.31%) patients. They were categorized into normal and mildly deranged groups.
Eleven (65%) patients had mildly deranged INR, and 6 (35%) patients had restoration of INR back to normal after administration of repeat doses of ASV and blood products.
aPTT was performed in all 58 patients on admission. They were categorized into normal (< 30 seconds) and deranged (>30 seconds) groups. Of the 58 patients, 48 (83%) had deranged aPTT, and 11 (17%) had normal aPTT.
A statistically significant correlation was observed between PT and aPTT values on admission (P = 0.008). The values of INR and aPTT were correlated with each other on admission, which was statistically significant (P = 0.008).
A statistically significant correlation was found between the total number of ASV vials administered and PT, INR, and aPTT values on admission (P = 0.004; Table 1).
A significant correlation was found between the INR levels and the total number of days spent in the ICU on admission and after 12 hours of admission (P = 0.042; Table 2).

The correlation between other coagulation parameters was not statistically significant. A significant correlation was found between the total number of FFP units administered and PT and INR on admission (P < 0.05; Table 3). The correlation between the total number of FFP units administered and aPTT was not statistically significant (P = 0.832). The correlation between the total number of blood components administered and PT was statistically significant on admission (P = 0.001). The correlation between the total number of blood components administered and INR was statistically significant on admission (P ≤ 0.001). The correlation between the total number of blood components administered and aPTT was not statistically significant (P = 0.785).


Discussion
In our study, the majority of the patients were male (69%), which is consistent with the study by Agrawal et al (64%) and Hariprasad et al (75%) (17, 32). However, Karthikeyan et al showed a female predominance (51%) (30).
In our study, 81% of patients showed prolongation of PT on admission. Agrawal et al showed prolongation of PT-INR in 26% of cases, while Harshvardhana et al showed prolongation of PT (>15 seconds) in 56% of patients (32, 33). Karthikeyan et al showed prolongation of PT (PT > 17 seconds) in 75.6% of patients (30).
Ireland et al showed deranged INR (>1.2) in 86% of cases on admission (34). Prolongation of PT significantly correlated with the total number of ASV vials administered. Patients with deranged PT on admission required a greater number of ASV vials to achieve normal coagulation. Ramamurthy et al also showed the increased requirement of ASV with the prolongation of PT (33).
Prothrombin time >17 seconds was also associated with the increased requirement of FFP in the current study. Similarly, Harshvardhana et al showed that 136 FFP units were required for patients with PT of more than 15 seconds (33).
In the current study, PT-INR was performed on admission. Of the 58 patients, 26 (45%) had mildly deranged INR (1.2- 4.0), 3 (5%) had moderately deranged INR, and 13 (22%) had severely deranged INR on admission. Agrawal et al showed prolongation of INR in 26% of cases, while Hariprasad et al showed prolongation of INR (>1.2) in 85% of patients (17, 32). Ireland et al showed deranged INR (>1.2) in 86% of cases on admission (34).
In our study, it was observed that the administration of ASV resulted in the restoration of INR to normal levels in some cases. However, in a significant proportion of patients (specifically, 77% of those whose INR was reassessed), the INR remained prolonged even after 12 hours of admission. Prolongation of INR beyond 12 hours was seen in 3.7% of cases in the study by Agrawal et al (32). Ireland et al showed deranged INR beyond 12 hours in 14% of cases (34). Of the 17 patients, 11 (65%) required a repeat INR measurement 24 hours after admission, showing that the INR values were mildly deranged. However, in the existing literature, the prolongation of INR has only been studied for up to 12 hours. The international normalized ratio on admission and 12 hours after admission demonstrated a significant correlation with the total number of days spent in the ICU. Mean INR on admission and 12 hours after admission was 8.5 and 1.5, respectively, for 4 days in the ICU, whereas it was 1.4 and 1.3 for 1 day in the ICU. This result was significant as morbidity can be predicted significantly by following up INR 12 hours after admission. In our study, a significant correlation was found between the derangement of INR on admission and the total number of days spent in the ICU. Patients with severely deranged INR had a significantly longer duration of stay in the ICU compared with those with mildly deranged INR values. These results are consistent with the results of Hariprasad et al (17). In our study, a significant correlation was found between the derangement of INR on admission and the total number of ASV vials administered. This correlation was also significant in the studies by Ramamurthy et al and Harshvardhan et al (29, 35). Prolonged INR is thus an indication of a repeat dose of ASV and FFP.
In the current study, the total number of FFP units administered significantly correlated with INR on admission. Similarly, Harshvardhana et al found that 136 FFP units were required for 25 days of hospitalization days in patients with PT of more than 15 seconds. (33) In the study by Hariprasad et al, 112 patients with INR more than 1.5 also required FFP (17).
In our study, aPTT was prolonged in 83% of patients. Similar results were seen in the study by Patil et al (73.6%) and Agrawal et al (26.4%) (32, 35). In the current study, aPTT significantly correlated with the total number of ASV vials administered. Ramamurthy et al also showed that the prolongation of aPTT was associated with an increased requirement of ASV (29). In our study, prolonged aPTT was associated with increased days of hospitalization in the ICU. These results are consistent with the results of Harshvardhana et al (33).
Implications of the study
This study offers practical guidelines for the use of coagulation tests in the management of snakebite patients. It provides insights into the optimal timing and frequency of conducting coagulation tests in these patients, as well as predicting the need for ASV and blood products such as FFP.
Limitations of the study
In the present study, the proportion of mild to moderate cases was more than severe cases.
Conclusion
Coagulation parameters (ie, PT, INR, and aPTT) can be reliably used for predicting the requirement for ASV, FFP, other blood products, and duration of ICU stay. Thus, coagulation profile estimation can be used in predicting snakebite patients’ prognosis. To enhance the prognostic value of coagulation tests, it is recommended to repeat these tests at 12-hour intervals in patients with deranged coagulation profiles.
Acknowledgement
We acknowledge the Head of the Department of Pathology and MIMER Medical College for guidance and constant support.
Funding sources
No funding was used for this study.
Ethical statement
All procedures performed in the current study were approved by Institutional review board (IRB) of MIMER Medical College, Talegaon (D) (approval number- MUHS/Medical/MUHS-020660/2019) in accordance with the1964 Helsinki declaration and its later amendments.
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
The authors declare that they have no potential conflict of interest to disclose.
Author contributions
1. GSM contributed significantly in conceptualization, methodology, project administration, managing resources, supervising the project, validation visualization, writing original draft and writing review.
2. SPS has contributed significantly in data curation, formal analysis, conducting investigations, using software for statistical analysis and writing original draft.
Research Article: Original Paper |
Subject:
Laboratory Sciences
Received: 2022/09/22 | Accepted: 2023/02/15 | Published: 2023/10/2 | ePublished: 2023/10/2
Received: 2022/09/22 | Accepted: 2023/02/15 | Published: 2023/10/2 | ePublished: 2023/10/2
References
1. Redewad N, Bhaisare SD, Bansod YV, Hire R. Management and outcome study of snake bite cases in central India. Sch J Appl Med Sci. 2014;2(01):435-41. [View at Publisher] [Google Scholar]
2. Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):e218. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. World Health Organization. Rabies and envenomings. A neglected public health issue: Report of a consultative meeting. Geneva:WHO;2007. [View at Publisher] [Google Scholar]
4. Global Burden of Disease Collaborative Network. Global Burden of Disease study 2016 (GBD 2016) results. Seattle, WA: Institute for Health Metrics and Evaluation; 2017 (http://ghdx.healthdata.org/gbd-results-tool, accessed 27 February 2018).
5. Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:17063.
https://doi.org/10.1038/nrdp.2017.79 [View at Publisher] [DOI] [Google Scholar]
6. Peden M, Oyegbite K, Ozanne-Smith J, Hyder AA, Branche C, Fazlur Rahman AKM, et al., editors. World report on child injury prevention. Geneva: World Health Organization;2008. [View at Publisher] [Google Scholar]
7. HS Bawaskar, Bawaskar PH. Snake bite Poisoning. J Mahatma Gandhi Inst Med Sci 2015;20(1):5-14. [View at Publisher] [DOI] [Google Scholar]
8. Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: A disease of poverty. PLoS Negl Trop Dis. 2009;3(12):e569. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5(4):e1018. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76(5):515-24. [View at Publisher] [Google Scholar]
11. Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake Bite in South Asia: A Review. PLoS Negl Trop Dis. 2010;4(1):e603. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Suleman MM, Shahab S, Rab MA. Snake bite in the Thar Desert. J Pak Med Assoc. 1998;48(10):306-8. [View at Publisher] [Google Scholar]
13. Ariaratnam CA, Sheriff MH, Theakston RD, Warrell DA. Distinctive epidemiologic and clinical features of common krait (Bungarus caeruleus) bites in Sri Lanka. Am J Trop Med Hyg. 2008;79(3):458-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Kirte RC, Wahab SN, Bhathkule PR. Record based study of snake bite cases admitted at Shri Vasantrao Naik government medical college & hospital, Yavatmal (Maharashtra). Indian J Public Health. 2006;50(1):35-7. [View at Publisher] [Google Scholar]
15. Hati AK, Mandal M, De MK, Mukherjee H, Hati RN. Epidemiology of snake bite in District of Burdwan, West Bengal. J Indian Med Assoc. 1992;90(6):145-7. [View at Publisher] [Google Scholar]
16. Afsheen Ishfaq, Faran Maqbool, Saima Humayun Toor, Syed Irfan Ahmed. Hematotoxicity in patients with snake bite. J Rawalpindi Med Coll. 2014;18(1):20-22. [Google Scholar]
17. Hariprasad S,Sukhani N. Assessment of clinical parameters among patients with snake poison induced coagulopathy. Int J Adv Med. 2018;5(6):1374-9. [View at Publisher] [DOI] [Google Scholar]
18. Wedasingha S, Isbister G, Silva A. Bedside Coagulation Tests in Diagnosing Venom-Induced Consumption Coagulopathy in Snakebite. Toxins (Basel). 2020;12(9):583. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Warrell DA. Snake bite. Lancet. 2010;375(9708):77-88. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Berling I, Isbister GK. Hematologic effects and complications of snake envenoming. Transfus Med Rev. 2015;29(2):82-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Maduwage K, Isbister GK. Current Treatment for Venom-Induced Consumption Coagulopathy Resulting from snakebite. Plos Negl Trop Dis. 2014;8(10):e3220. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Isbister GK. Snakebite doesn't cause disseminated intravascular coagulation: Coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost. 2010; 36(4):444-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Bick RL. Disseminated intravascular coagulation, current concepts of etiology, pathophysiology, diagnosis and treatment. Hematol Oncol Clin North Am. 2003;17(1):149-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Kalantri S, Singh A, Joshi R, Malamba S, Ho C, Ezoua J, et al. Clinical predictors of in-hospital mortality in patients with snake bite: a retrospective study from a rural hospital in central India. Trop Med Int Health. 2006;11(1):22-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Singh Sujit, Singh G. Snake bite: Indian Guidelines and Protocol. Apiindia [internet]. 2013;chap 94;Sec 12:424-6. [View at Publisher] [Google Scholar]
26. Simpson ID. A study of current knowledge base in treating snake bite among doctors in high risk countries of India and Pakistan: does snake bite treatment training reflect local requirements? Trans R Soc Trop Med Hyg. 2008;102(11):1108-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. National snakebite management protocol, India. (2008). [online] Avaialable at: www://mohfw.nic.in (Directorate General of Health and Family Welfare, Ministry of Health and Family Welfare, India). [View at Publisher] [Google Scholar]
28. Warrell DA. WHO/SEARO Guidelines for the Clinical Management of Snakebite in the Southeast Asian Region. SE Asian J Trop Med Pub Health. 1999;30(Suppl1):1-85. [View at Publisher] [Google Scholar]
29. Ramamurthy P, Kuruchatti DP, Sunil KN, Huggi V. Kumar S. A study on coagulation profile and its prognostic significance in patients with snake envenomation. J Evol Med Dent Sci. 2014;3(51): 11959-65. [View at Publisher] [DOI] [Google Scholar]
30. Navaneetham K, Ganesan K. Correlation of platelet count with outcomes in snake bite victims with systemic envenomation. MedPulse International Journal of Medicine. 2018;7(1):20-24. [View at Publisher] [Google Scholar]
31. Bain BJ, Bates I, Laffan MA. Dacie and lewis practical haematology e-book. 12th ed. Elsevier Health Sci;2016:11. [View at Publisher] [Google Scholar]
32. Agarwal S, Prasad CS, Kumar MLH, Kumar U. Haematological and Coagulation Profile in Snake Envenomation. J Clin Biomed Sci. 2014;4(4):361-4. [View at Publisher] [DOI] [Google Scholar]
33. Punde DP. Management of snake-bite in rural Maharashtra: a 10-year experience. Natl Med J India. 2005;18(2):71-5. [View at Publisher] [Google Scholar]
34. Ireland G, Brown SG, Buckley NA, Stormer J, Currie BJ, White J, et al. Australian Snakebite Project Investigators. Changes in serial laboratory test results in snakebite patients: when can we safely exclude envenoming? Med J Aust. 2010;193(5):285-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Harshavardhana HS, Pasha I, Prabhu NS, Ravi P. Snake Bite Induced Coagulopathy: A Study of Clinical Profile and Predictors of Poor Outcome. Int J Sci Study. 2014;2(1):2-5. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







