Volume 17, Issue 5 (Sep-Oct 2023)
mljgoums 2023, 17(5): 4-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adeniyi T, Moronkeji A, Ekundina V. Histological evaluation of the liver, kidney, and testes of adult male Wistar rats exposed to heavy metals-contaminated waterways. mljgoums 2023; 17 (5) :4-8
URL: http://mlj.goums.ac.ir/article-1-1501-en.html
URL: http://mlj.goums.ac.ir/article-1-1501-en.html
1- Department of Medical Laboratory Science, Faculty of Allied Health Sciences, University of Medical Sciences Ondo, Ondo City, Nigeria. PMB 563, Laje Road, Ondo City, Ondo State, Nigeria
2- Department of Medical Laboratory Science, Faculty of Allied Health Sciences, University of Medical Sciences Ondo, Ondo City, Nigeria. PMB 563, Laje Road, Ondo City, Ondo State, Nigeria ,amoronkeji@unimed.edu.ng
3- Department of Medical Laboratory Science, Faculty of Allied Health Sciences, College of Medicine and Health Sciences, Afe Babalola Universit KM 8.5, Afe Babalola Way, Ado-Ekiti, Nigeria
2- Department of Medical Laboratory Science, Faculty of Allied Health Sciences, University of Medical Sciences Ondo, Ondo City, Nigeria. PMB 563, Laje Road, Ondo City, Ondo State, Nigeria ,
3- Department of Medical Laboratory Science, Faculty of Allied Health Sciences, College of Medicine and Health Sciences, Afe Babalola Universit KM 8.5, Afe Babalola Way, Ado-Ekiti, Nigeria
Full-Text [PDF 875 kb]
(1594 Downloads)
| Abstract (HTML) (5999 Views)
Full-Text: (1889 Views)
Introduction
Water plays an important role in the survival of all organisms, including humans, for food production and economic development (1). Human activities have negatively impacted the environment by introducing toxic waste to the environment via large amounts of heavy metal pollutants, which are being discharged through rivers or via atmospheric deposition into the aquatic environment (2,3). Various activities of humans connected to urbanization, population growth, industrial production, climate change, and other factors reportedly impact the quality of water and often result in water pollution, which is deleterious to the well-being of humans (1). Heavy metals can be derived from natural geological sources or anthropogenic sources, which include smelting, mining, agriculture, aquaculture, and industrial sewage (4). Heavy metals negatively impact the marine environment due to their biotoxicity and non-degradability (5,6). Non-essential metals such as cadmium (Cd) have also been implicated in hurting the growth and development of organisms at low levels (6,7). Heavy metals in the aquatic environment can bioaccumulate in aquatic organisms and biomagnify through the food chain (8). Regular consumption of dietary seafood remains a major route through which heavy metals are absorbed in humans, and the exposure to which is associated with various health problems, including organ damage, endocrine disruption, cancer, and neurological impacts (9). Akinola and Ekiyoyo (10) previously reported Cd pollution along River Ribila located in Odo-nla village off Sagamu road, Ikorodu, Lagos State, Nigeria (10). Haque et al. (11) also reported pollution in the Ganges River, China (11). Soil contamination with heavy metals can ultimately lead to soil pollution and the cultivated plants that are a ready food source for humans as well as other organisms in the food chain. The release of garbage, sewage, and oil spills, regardless of their assimilative capabilities, continually threatens the quality of water in urban environments, and the increased industrial wastes from factories constitute significant sources of toxic pollutants to the water body (12). Food contamination remains a major route of exposure to heavy metals, and increasing dietary heavy metals have been implicated in the development of several diseases, especially after several years of exposure (13). The monitoring of river water quality is important, especially when habitant utilizes water for household purposes such as drinking, cooking, and bathing (14). This study evaluated the effect of heavy metals acquired from the waterways on the vital organs of experimental rats. Furthermore, histopathological analysis was conducted on the kidneys, liver, and testes of adult male Wistar rats upon chronic administration of water from the rivers.
Methods
A cross-sectional surveillance and animal experimentation type of study was conducted. The frequency or prevalence of heavy metal contamination was obtained as the empirical measurement, which enabled the collection of data and provided a basis for making a conclusion, which was then used in the animal experimentation study comprising both control and treatment groups.
Ethical statement
The experimental protocol and procedures used in this study were approved by the College of Medicine Research Ethics Committee, Directorate of Research and Publications, College of Medicine, University of Nigeria, Enugu Campus, State, Nigeria, with protocol number 025/02/2017. This approval is consistent with those set down by the National Institute of Health (NIH) in the “Guide to the Care and Use of Animals in Research and Teaching” (15).
Measurement of heavy metals
Lead (II) acetate trihydrate (Pb(CH3CO2).3H2O), Mercury (II) thiocyanate (Hg(SCN)2), Cadmium acetate dehydrate (Cd(CH3CO2).2H2O), Chromium (III) oxide (Cr2O3) were procured from Sigma-Aldrich (USA). 0.009 g of Pb(CH3CO2).3H2O, 0.001 g of Hg(SCN)2, 0.045 g of Cd(CH3CO2).2H2O and 0.318 g of Cr2O3 were weighed using Meltler sensitive weighing balance and dissolved in 1 liter of double-distilled demineralized water to form 0.009 mg of Pb(CH3CO2).3H2O, 0.001 mg of Hg(SCN)2, 0.045 mg of Cd(CH3CO2).2H2O and 0.318 mg of Cr2O3 per liters solutions, respectively. This was based on the empirical measurement of heavy metals obtained in the waterways of the study area and reported by Adeniyi et al. (16).
Animals and diet
Seventy (70) first filial (F1) generations inbred adult male Wistar rats (Rattus norvergicus) with an average weight of about 150-180 grams were procured from the Institute for Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Nigeria. They were allowed to acclimatize for 14 days and were fed with pelletized rat feed and water ad libitum throughout acclimatization before use. The rats were housed in plastic cages. They were kept in standard laboratory conditions under a natural light-dark cycle at room temperature and maintained on standard laboratory rat pellets and given water ad libitum. Seventy (70) rats were divided into seven groups of ten animals, each selected by simple randomization using the method of. Hau et al. (17). The duration of treatment lasted 65 days and the animals were euthanized by cervical dislocation. The liver, kidneys, and testes were excised and immediately transferred into 10% neutral buffered formalin for adequate fixation for 72 hours. The fixed tissues were histologically processed using the Thermo Scientific Spin Tissue Processor, STP120, Frankfurt, Germany. The tissue sections obtained were stained using the Haematoxylin and Eosin (H&E) staining technique and evaluated microscopically. Histological analysis was carried out using the H&E staining technique to demonstrate the general architectural profile of the liver, kidneys, and testes. The stained sections were viewed and photographed with an Olympus U-D03 microscope.
Table 1. Grouping and treatment of animals

Results
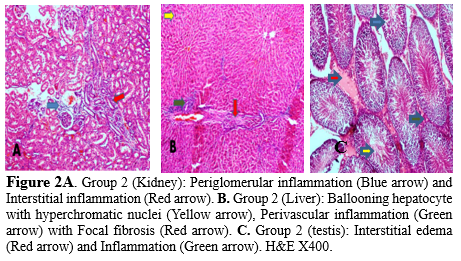
Histological studies across the various exposed groups revealed varying cytopathic features in the organs of the experimental animals. The kidney, liver, and testes of the control rats were devoid of pathological lesions (Figure 1), while the kidney, liver, and testes of the rats exposed to the pooled sampled water or Lead (II) acetate trihydrate showed a variety of histological changes, including periglomerular inflammation and dilated blood vessels in the kidney, perivascular inflammation and fibrosis in the liver, along with interstitial edema and inflammation of the testes (Figures 2,3).

Rats administered with Mercury (II) thiocyanate displayed sinusoidal dilatation and distention in their livers (Figure 4), while focal hypospermatogenesis was evident in rats administered with either Cadmium acetate dehydrate, Chromium (III) oxide, or a combination of the heavy metals (Figures 5-7).


Discussion
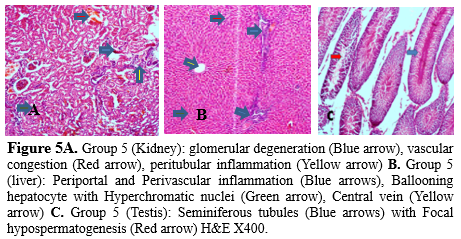
Most rivers and reservoirs are prone to pollution because of the indiscriminate disposal of domestic, agricultural, and industrial wastes into water bodies (18). Due to environmental contamination brought on by industrial processes and products, human exposure to heavy metals like mercury (Hg), lead (Pb), and cadmium have considerably increased over the past 50 years (19). Soil and water pollution has significantly impacted food safety and quality and remains a major concern to human health (20). This study utilized the highest concentration of identified heavy metals in fifteen rivers sampled in different seasons across three geological zones in Kwara state, Nigeria (16), to further observe histopathological changes in organs of rats administered with water containing the identified heavy metals. The anthropogenic activities of humans have been indicated to predispose humans to various maladies due to the release of toxic substances into the environment, with the incidence of cancers and infertility increasing in the last fifty years (21). Additionally, the ratio of heavy metal’s beneficial and harmful effects depends on their concentrations within living cells; thus requiring that the levels of metal ions have to be maintained within an appropriate range to prevent nutritional deficiencies, whereas higher concentrations can cause health concerns (22). Mercury (Hg) can induce organ toxicity in the central nervous system, hepatotoxicity, gastrointestinal alterations, and renal dysfunction with disruption of macromolecules or mechanism of actions involving aquaporin mRNA reduction, glutathione peroxidase inhibition, enzyme inhibition, production of reactive oxygen species, and Thiol binding (23,24). Lead can also induce organ toxicity, including central nervous system (CNS) injury, Hematological changes such as anemia (25), pulmonary dysfunction, reduced pulmonary function (26), Liver damage as well as cardiovascular dysfunction with the mechanism of action involving enhanced levels of inflammatory cytokines such as interleukin -1 (IL-1), tumor necrosis factor-alpha (TNF-α) and IL-6 in the CNS (27), Increased serum Endothelin 1 (ET-1), nitric oxide (NO), and erythropoietin (EPO) levels, Inactivation of Delta-aminolevulinic acid dehydratase (δ-ALAD) and ferrochelatase (inhibition of heme biosynthesis), reduced Glutathione (GSH), Superoxide dismutase (SOD), catalase (CAT), and Glutathione peroxidase (GPx) levels (28) . The liver, kidneys, and testes of the control group were devoid of pathological lesions (Figure 1 a, b, c). Pathological lesions in the organs of rats administered with Cadmium, Chromium, and a combined (Lead, Mercury, Cadmium, and Chromium) cocktail of the metals used in this study showed significant lesions when compared with organs of rats administered with pooled sampled water only. Chromium-induced toxicity has been documented by (29,30) with the mechanism of damage involving DNA damage, genomic instability, oxidative stress, and reactive oxygen species (ROS) generation. Gastrointestinal disorders, dermal diseases, kidney dysfunction, as well an increase in the occurrence of cancers, including bladder, kidneys, lungs, larynx, testicular, bone, and thyroid. Heavy metal concentration in the blood is of significant consideration for public health (19), and excessive dietary intake of lead is implicated in several cancers (31). Matouke and Lami (32) reported elevated levels of Cd, and Chromium (Cr), in Clarias gariepinus, further suggesting that consuming these fishes could predispose humans to various chronic diseases. Pb, Cd, and Hg are natural heavy metals found in geological formations in the earth’s crust with characteristic high densities (>5g/cm3) (33) with exposure to these chemicals confirmed to be toxic to the reproductive systems; thus, requiring standard public health monitoring and interventions (34). Several organs, such as the Kidney, Liver, and Lung, are damaged by cadmium exposure, metabolic syndromes associated with Zn and Cu, degenerative bone disease, and various cancers have also been reported (22). The mechanism of action by which cadmium induces these disruptions includes miRNA expression dysregulation (35), endoplasmic reticulum stress, Cd-MT absorption by the kidneys, dysregulation of Ca, Zn, and Fe homeostasis, low serum parathormone (PTH) levels, ROS generation, and altered phosphorylation cascades (36,37). Environmental and occupational exposure to heavy metals remains a global health issue as it alters the biological system, thus predisposing humans to infertility and affecting 15% of couples of reproductive age (21). The toxic mechanisms include the disruption of cell signaling pathways, oxidative stress, altered gene expression, epigenetic regulation of gene expression, apoptosis, and disruption of the testis–blood barrier, inflammation, endocrine disruption, and ion mimicry (38). Water pollution remains a global potential threat to humans and animals that interact with the aquatic environment (39). Aquatic animals can accumulate pollutants directly from contaminated water, with fish storing these pollutants in various organs (18). These contaminants could bioaccumulate and transcend into human diets, thus leading to several chronic diseases (8). Stout et al. (40) reported that hexavalent chromium orally administered induced small intestinal tumors in B6C3F1 mice and tumors of the oral cavity in rats (40). The kidneys of rats administered with the pooled water sample showed the presence of periglomerular inflammation and distended blood vessels coupled with interstitial inflammation (Figure 2a). Deterioration of water bodies and the genotoxic potential of pollutants in rivers and reservoirs in north-central Ilorin has also been reported by Anifowoshe et al. (18). Rats administered with the pooled water sample displayed localized fibrosis and perivascular inflammation in their liver (18). Also, hyperchromatic nuclei and ballooning hepatocytes were evident (Figure 2b). This conforms with a study by Oladipo et al. (41), who reported distorted liver tissue architecture with infiltration and uneven sinusoidal distribution in rats exposed to automobile waste leachates in Ilorin (41). The testes of rats in this category showed the presence of interstitial edema and inflammation when compared with the control (Figure 2c). This is similar to the report of Oladipo et al. (41), who described cytopathic changes such as reduced vascular lumen, vacuolation of tubule germinal epithelium, well as degeneration of the tubular epithelium accompanied by acute vacuolation and atrophy of the germinal epithelium in the testes of rats exposed to leachates in Ilorin West local government area of Kwara state (41). This change has the potential to induce infertility in the animals. Akintunde et al. (42) also documented idiopathic infertility in male Wistar rats exposed to leachates from batteries of electronic devices via induction of peroxidation, impaired cell membrane, and reduced sperm membrane fluidity leading to injury of spermatozoa in the testes of rats (42). The kidneys’ histological examination of the rats administered with lead-containing water showed tubular degeneration, vascular congestion, and glomerular degeneration (Figure 3a), while the liver showed ballooning hepatocytes with hyperchromatic nuclei with congested central vein coupled with periportal inflammation (Figure 3b). Jarrar and Taib (43) reported that these features are consistent with lead toxicity (43). The testes of rats in this group were characterized by the presence of interstitial edema and inflammation (Figure 3c). Yakubu and Omar (44) reported reduced sperm count and motility as well as testicular distortion of the seminiferous tubules and cellular degeneration in the testes of adult male rats exposed to groundwater samples and leachates from Gbagede dumpsite, Amoyo, Kwara State, Nigeria (44). The administration of mercury revealed a kidney characterized by severe glomerular degeneration coupled with vascular distortion and congestion (Figure 4a), while the liver revealed periportal and perivascular inflammation coupled with sinusoidal dilation and distention (Figure 4b). The testes of rats in this category appeared moderately normal; however, edema was observed within the tissue (Figure 4c). This is consistent with the findings of Akintunde et al. (42), who indicated that leachate containing lead and cadmium might be harmful to the testicular cell membrane on exposure to the mixed metal, which could serve as an agent of testicular damage and infertility in males (42). The kidney of cadmium administered group showed the presence of glomerular degeneration, vascular congestion as well as peritubular inflammation (Figure 5a), while the liver showed ballooning hepatocytes with hyperchromatic nuclei; periportal and perivascular inflammation was also evident (Figure 5b).
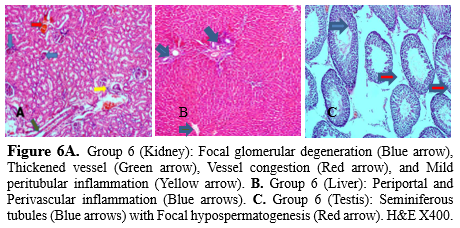
The testes of the exposed rats revealed hypospermatogenesis (Figure 5c). Yakubu and Omar (44) also reported the presence of groundwater leachate from Gbagede dumpsite in Amoyo, Kwara State, with the testicular histology revealing distortion of the seminiferous tubules and cellular degeneration in groups exposed to groundwater and leachates (44). Oladipo et al. (41) documented the structural abnormalities in the kidney, liver, and testes of albino mice exposed to automobile waste leachate. The kidneys of rats administered with the chromium-containing solution revealed focal glomerular degeneration with thickened vessels as well as congestion coupled with mild inflammation (Figure 6a), while the liver revealed peripheral and perivascular inflammation (Figure 6b). The testes of rats in this group were characterized by focal hypospermatogenesis (Figure 6c). The occurrence of cadmium in nature is low, and it is mainly associated with the ores of lead, zinc, and copper, with a biological half-life in humans ranging from 7 to 26 years in the kidney and 3 to 4 months in the blood (33).
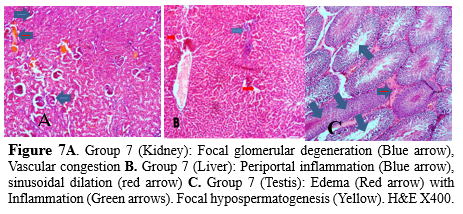
Long-term exposure to cadmium through the air, water, soil, and food may be associated with cancer and organ system toxicity, such as skeletal, urinary, reproductive, cardiovascular, central and peripheral nervous, and respiratory systems (45). Thorne et al. (46) previously reported a failure in spermiation in a single low dose of cadmium administration in adult male Wistar rats, while De Souza Predes et al. (47) also documented that slight variation in cadmium doses could cause a surge in testicular damage, to include vacuolization of the seminiferous epithelium accompanied by diminished height and azoospermia in rats (46,47) further buttressing our findings in this study. The final group administered with the combined heavy metal solution (Pb, Hg, Cd, and Cr) revealed focal glomerular degeneration and vascular congestion in the kidneys, with the interstitial spaces appearing normal and not inflamed (Figure 7a). However, periportal inflammation, sinusoidal dilation, and mild congestion of the central vein were evident in the liver of rats in this group (Figure 7b), while the testes of rats were mildly inflamed with focal hypospermatogenesis evident (Figure 7c).
Conclusion
This study revealed the effects of variations in the water quality on vital organs of the body. It is relevant for the control and management of heavy metals in Kwara state, Nigeria. Individuals who utilize water directly from these rivers for household purposes must take precautions to prevent the possible adverse health effects that may be associated with these metals. Additionally, regulatory agencies must constantly monitor activity on these water bodies, and regulations that strictly forbid dumping waste in water should be enforced.
Acknowledgement
Not applicable.
Funding sources
The present research did not receive any financial support.
Ethical statement
The study was performed in accordance with the “the National Institute of Health (NIH) in the “Guide to the Care and Use of Animals in Research and Teaching published by the National Academic Press (Eighth edition, International Standard Book Number-13: 978-0-309-15400-0). The experimental protocol and procedures used in this study were approved by the College of Medicine Research Ethics Committee, Directorate of Research and Publications, College of Medicine, University of Nigeria, Enugu Campus, State, Nigeria, with protocol number 025/02/2017.
Conflicts of interest
The authors declare that there is not any conflict of interest regarding the publication of this manuscript.
Author contributions
The authors confirm contribution to the paper as follows: Study conception and design: Temidayo Daniel Adeniyi: Data collection: Temidayo Daniel Adeniyi, Akinpelu Moronkeji; Analysis and interpretation of results: Akinpelu Moronkeji, Temidayo Daniel Adeniyi, Victor Olukayode Ekundina; Draft manuscript preparation: Akinpelu Moronkeji. All authors reviewed and approved the final version of the manuscript.
Water plays an important role in the survival of all organisms, including humans, for food production and economic development (1). Human activities have negatively impacted the environment by introducing toxic waste to the environment via large amounts of heavy metal pollutants, which are being discharged through rivers or via atmospheric deposition into the aquatic environment (2,3). Various activities of humans connected to urbanization, population growth, industrial production, climate change, and other factors reportedly impact the quality of water and often result in water pollution, which is deleterious to the well-being of humans (1). Heavy metals can be derived from natural geological sources or anthropogenic sources, which include smelting, mining, agriculture, aquaculture, and industrial sewage (4). Heavy metals negatively impact the marine environment due to their biotoxicity and non-degradability (5,6). Non-essential metals such as cadmium (Cd) have also been implicated in hurting the growth and development of organisms at low levels (6,7). Heavy metals in the aquatic environment can bioaccumulate in aquatic organisms and biomagnify through the food chain (8). Regular consumption of dietary seafood remains a major route through which heavy metals are absorbed in humans, and the exposure to which is associated with various health problems, including organ damage, endocrine disruption, cancer, and neurological impacts (9). Akinola and Ekiyoyo (10) previously reported Cd pollution along River Ribila located in Odo-nla village off Sagamu road, Ikorodu, Lagos State, Nigeria (10). Haque et al. (11) also reported pollution in the Ganges River, China (11). Soil contamination with heavy metals can ultimately lead to soil pollution and the cultivated plants that are a ready food source for humans as well as other organisms in the food chain. The release of garbage, sewage, and oil spills, regardless of their assimilative capabilities, continually threatens the quality of water in urban environments, and the increased industrial wastes from factories constitute significant sources of toxic pollutants to the water body (12). Food contamination remains a major route of exposure to heavy metals, and increasing dietary heavy metals have been implicated in the development of several diseases, especially after several years of exposure (13). The monitoring of river water quality is important, especially when habitant utilizes water for household purposes such as drinking, cooking, and bathing (14). This study evaluated the effect of heavy metals acquired from the waterways on the vital organs of experimental rats. Furthermore, histopathological analysis was conducted on the kidneys, liver, and testes of adult male Wistar rats upon chronic administration of water from the rivers.
Methods
A cross-sectional surveillance and animal experimentation type of study was conducted. The frequency or prevalence of heavy metal contamination was obtained as the empirical measurement, which enabled the collection of data and provided a basis for making a conclusion, which was then used in the animal experimentation study comprising both control and treatment groups.
Ethical statement
The experimental protocol and procedures used in this study were approved by the College of Medicine Research Ethics Committee, Directorate of Research and Publications, College of Medicine, University of Nigeria, Enugu Campus, State, Nigeria, with protocol number 025/02/2017. This approval is consistent with those set down by the National Institute of Health (NIH) in the “Guide to the Care and Use of Animals in Research and Teaching” (15).
Measurement of heavy metals
Lead (II) acetate trihydrate (Pb(CH3CO2).3H2O), Mercury (II) thiocyanate (Hg(SCN)2), Cadmium acetate dehydrate (Cd(CH3CO2).2H2O), Chromium (III) oxide (Cr2O3) were procured from Sigma-Aldrich (USA). 0.009 g of Pb(CH3CO2).3H2O, 0.001 g of Hg(SCN)2, 0.045 g of Cd(CH3CO2).2H2O and 0.318 g of Cr2O3 were weighed using Meltler sensitive weighing balance and dissolved in 1 liter of double-distilled demineralized water to form 0.009 mg of Pb(CH3CO2).3H2O, 0.001 mg of Hg(SCN)2, 0.045 mg of Cd(CH3CO2).2H2O and 0.318 mg of Cr2O3 per liters solutions, respectively. This was based on the empirical measurement of heavy metals obtained in the waterways of the study area and reported by Adeniyi et al. (16).
Animals and diet
Seventy (70) first filial (F1) generations inbred adult male Wistar rats (Rattus norvergicus) with an average weight of about 150-180 grams were procured from the Institute for Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Nigeria. They were allowed to acclimatize for 14 days and were fed with pelletized rat feed and water ad libitum throughout acclimatization before use. The rats were housed in plastic cages. They were kept in standard laboratory conditions under a natural light-dark cycle at room temperature and maintained on standard laboratory rat pellets and given water ad libitum. Seventy (70) rats were divided into seven groups of ten animals, each selected by simple randomization using the method of. Hau et al. (17). The duration of treatment lasted 65 days and the animals were euthanized by cervical dislocation. The liver, kidneys, and testes were excised and immediately transferred into 10% neutral buffered formalin for adequate fixation for 72 hours. The fixed tissues were histologically processed using the Thermo Scientific Spin Tissue Processor, STP120, Frankfurt, Germany. The tissue sections obtained were stained using the Haematoxylin and Eosin (H&E) staining technique and evaluated microscopically. Histological analysis was carried out using the H&E staining technique to demonstrate the general architectural profile of the liver, kidneys, and testes. The stained sections were viewed and photographed with an Olympus U-D03 microscope.
Table 1. Grouping and treatment of animals

Results
Histological studies across the various exposed groups revealed varying cytopathic features in the organs of the experimental animals. The kidney, liver, and testes of the control rats were devoid of pathological lesions (Figure 1), while the kidney, liver, and testes of the rats exposed to the pooled sampled water or Lead (II) acetate trihydrate showed a variety of histological changes, including periglomerular inflammation and dilated blood vessels in the kidney, perivascular inflammation and fibrosis in the liver, along with interstitial edema and inflammation of the testes (Figures 2,3).


Rats administered with Mercury (II) thiocyanate displayed sinusoidal dilatation and distention in their livers (Figure 4), while focal hypospermatogenesis was evident in rats administered with either Cadmium acetate dehydrate, Chromium (III) oxide, or a combination of the heavy metals (Figures 5-7).





Discussion
Most rivers and reservoirs are prone to pollution because of the indiscriminate disposal of domestic, agricultural, and industrial wastes into water bodies (18). Due to environmental contamination brought on by industrial processes and products, human exposure to heavy metals like mercury (Hg), lead (Pb), and cadmium have considerably increased over the past 50 years (19). Soil and water pollution has significantly impacted food safety and quality and remains a major concern to human health (20). This study utilized the highest concentration of identified heavy metals in fifteen rivers sampled in different seasons across three geological zones in Kwara state, Nigeria (16), to further observe histopathological changes in organs of rats administered with water containing the identified heavy metals. The anthropogenic activities of humans have been indicated to predispose humans to various maladies due to the release of toxic substances into the environment, with the incidence of cancers and infertility increasing in the last fifty years (21). Additionally, the ratio of heavy metal’s beneficial and harmful effects depends on their concentrations within living cells; thus requiring that the levels of metal ions have to be maintained within an appropriate range to prevent nutritional deficiencies, whereas higher concentrations can cause health concerns (22). Mercury (Hg) can induce organ toxicity in the central nervous system, hepatotoxicity, gastrointestinal alterations, and renal dysfunction with disruption of macromolecules or mechanism of actions involving aquaporin mRNA reduction, glutathione peroxidase inhibition, enzyme inhibition, production of reactive oxygen species, and Thiol binding (23,24). Lead can also induce organ toxicity, including central nervous system (CNS) injury, Hematological changes such as anemia (25), pulmonary dysfunction, reduced pulmonary function (26), Liver damage as well as cardiovascular dysfunction with the mechanism of action involving enhanced levels of inflammatory cytokines such as interleukin -1 (IL-1), tumor necrosis factor-alpha (TNF-α) and IL-6 in the CNS (27), Increased serum Endothelin 1 (ET-1), nitric oxide (NO), and erythropoietin (EPO) levels, Inactivation of Delta-aminolevulinic acid dehydratase (δ-ALAD) and ferrochelatase (inhibition of heme biosynthesis), reduced Glutathione (GSH), Superoxide dismutase (SOD), catalase (CAT), and Glutathione peroxidase (GPx) levels (28) . The liver, kidneys, and testes of the control group were devoid of pathological lesions (Figure 1 a, b, c). Pathological lesions in the organs of rats administered with Cadmium, Chromium, and a combined (Lead, Mercury, Cadmium, and Chromium) cocktail of the metals used in this study showed significant lesions when compared with organs of rats administered with pooled sampled water only. Chromium-induced toxicity has been documented by (29,30) with the mechanism of damage involving DNA damage, genomic instability, oxidative stress, and reactive oxygen species (ROS) generation. Gastrointestinal disorders, dermal diseases, kidney dysfunction, as well an increase in the occurrence of cancers, including bladder, kidneys, lungs, larynx, testicular, bone, and thyroid. Heavy metal concentration in the blood is of significant consideration for public health (19), and excessive dietary intake of lead is implicated in several cancers (31). Matouke and Lami (32) reported elevated levels of Cd, and Chromium (Cr), in Clarias gariepinus, further suggesting that consuming these fishes could predispose humans to various chronic diseases. Pb, Cd, and Hg are natural heavy metals found in geological formations in the earth’s crust with characteristic high densities (>5g/cm3) (33) with exposure to these chemicals confirmed to be toxic to the reproductive systems; thus, requiring standard public health monitoring and interventions (34). Several organs, such as the Kidney, Liver, and Lung, are damaged by cadmium exposure, metabolic syndromes associated with Zn and Cu, degenerative bone disease, and various cancers have also been reported (22). The mechanism of action by which cadmium induces these disruptions includes miRNA expression dysregulation (35), endoplasmic reticulum stress, Cd-MT absorption by the kidneys, dysregulation of Ca, Zn, and Fe homeostasis, low serum parathormone (PTH) levels, ROS generation, and altered phosphorylation cascades (36,37). Environmental and occupational exposure to heavy metals remains a global health issue as it alters the biological system, thus predisposing humans to infertility and affecting 15% of couples of reproductive age (21). The toxic mechanisms include the disruption of cell signaling pathways, oxidative stress, altered gene expression, epigenetic regulation of gene expression, apoptosis, and disruption of the testis–blood barrier, inflammation, endocrine disruption, and ion mimicry (38). Water pollution remains a global potential threat to humans and animals that interact with the aquatic environment (39). Aquatic animals can accumulate pollutants directly from contaminated water, with fish storing these pollutants in various organs (18). These contaminants could bioaccumulate and transcend into human diets, thus leading to several chronic diseases (8). Stout et al. (40) reported that hexavalent chromium orally administered induced small intestinal tumors in B6C3F1 mice and tumors of the oral cavity in rats (40). The kidneys of rats administered with the pooled water sample showed the presence of periglomerular inflammation and distended blood vessels coupled with interstitial inflammation (Figure 2a). Deterioration of water bodies and the genotoxic potential of pollutants in rivers and reservoirs in north-central Ilorin has also been reported by Anifowoshe et al. (18). Rats administered with the pooled water sample displayed localized fibrosis and perivascular inflammation in their liver (18). Also, hyperchromatic nuclei and ballooning hepatocytes were evident (Figure 2b). This conforms with a study by Oladipo et al. (41), who reported distorted liver tissue architecture with infiltration and uneven sinusoidal distribution in rats exposed to automobile waste leachates in Ilorin (41). The testes of rats in this category showed the presence of interstitial edema and inflammation when compared with the control (Figure 2c). This is similar to the report of Oladipo et al. (41), who described cytopathic changes such as reduced vascular lumen, vacuolation of tubule germinal epithelium, well as degeneration of the tubular epithelium accompanied by acute vacuolation and atrophy of the germinal epithelium in the testes of rats exposed to leachates in Ilorin West local government area of Kwara state (41). This change has the potential to induce infertility in the animals. Akintunde et al. (42) also documented idiopathic infertility in male Wistar rats exposed to leachates from batteries of electronic devices via induction of peroxidation, impaired cell membrane, and reduced sperm membrane fluidity leading to injury of spermatozoa in the testes of rats (42). The kidneys’ histological examination of the rats administered with lead-containing water showed tubular degeneration, vascular congestion, and glomerular degeneration (Figure 3a), while the liver showed ballooning hepatocytes with hyperchromatic nuclei with congested central vein coupled with periportal inflammation (Figure 3b). Jarrar and Taib (43) reported that these features are consistent with lead toxicity (43). The testes of rats in this group were characterized by the presence of interstitial edema and inflammation (Figure 3c). Yakubu and Omar (44) reported reduced sperm count and motility as well as testicular distortion of the seminiferous tubules and cellular degeneration in the testes of adult male rats exposed to groundwater samples and leachates from Gbagede dumpsite, Amoyo, Kwara State, Nigeria (44). The administration of mercury revealed a kidney characterized by severe glomerular degeneration coupled with vascular distortion and congestion (Figure 4a), while the liver revealed periportal and perivascular inflammation coupled with sinusoidal dilation and distention (Figure 4b). The testes of rats in this category appeared moderately normal; however, edema was observed within the tissue (Figure 4c). This is consistent with the findings of Akintunde et al. (42), who indicated that leachate containing lead and cadmium might be harmful to the testicular cell membrane on exposure to the mixed metal, which could serve as an agent of testicular damage and infertility in males (42). The kidney of cadmium administered group showed the presence of glomerular degeneration, vascular congestion as well as peritubular inflammation (Figure 5a), while the liver showed ballooning hepatocytes with hyperchromatic nuclei; periportal and perivascular inflammation was also evident (Figure 5b).
The testes of the exposed rats revealed hypospermatogenesis (Figure 5c). Yakubu and Omar (44) also reported the presence of groundwater leachate from Gbagede dumpsite in Amoyo, Kwara State, with the testicular histology revealing distortion of the seminiferous tubules and cellular degeneration in groups exposed to groundwater and leachates (44). Oladipo et al. (41) documented the structural abnormalities in the kidney, liver, and testes of albino mice exposed to automobile waste leachate. The kidneys of rats administered with the chromium-containing solution revealed focal glomerular degeneration with thickened vessels as well as congestion coupled with mild inflammation (Figure 6a), while the liver revealed peripheral and perivascular inflammation (Figure 6b). The testes of rats in this group were characterized by focal hypospermatogenesis (Figure 6c). The occurrence of cadmium in nature is low, and it is mainly associated with the ores of lead, zinc, and copper, with a biological half-life in humans ranging from 7 to 26 years in the kidney and 3 to 4 months in the blood (33).
Long-term exposure to cadmium through the air, water, soil, and food may be associated with cancer and organ system toxicity, such as skeletal, urinary, reproductive, cardiovascular, central and peripheral nervous, and respiratory systems (45). Thorne et al. (46) previously reported a failure in spermiation in a single low dose of cadmium administration in adult male Wistar rats, while De Souza Predes et al. (47) also documented that slight variation in cadmium doses could cause a surge in testicular damage, to include vacuolization of the seminiferous epithelium accompanied by diminished height and azoospermia in rats (46,47) further buttressing our findings in this study. The final group administered with the combined heavy metal solution (Pb, Hg, Cd, and Cr) revealed focal glomerular degeneration and vascular congestion in the kidneys, with the interstitial spaces appearing normal and not inflamed (Figure 7a). However, periportal inflammation, sinusoidal dilation, and mild congestion of the central vein were evident in the liver of rats in this group (Figure 7b), while the testes of rats were mildly inflamed with focal hypospermatogenesis evident (Figure 7c).
Conclusion
This study revealed the effects of variations in the water quality on vital organs of the body. It is relevant for the control and management of heavy metals in Kwara state, Nigeria. Individuals who utilize water directly from these rivers for household purposes must take precautions to prevent the possible adverse health effects that may be associated with these metals. Additionally, regulatory agencies must constantly monitor activity on these water bodies, and regulations that strictly forbid dumping waste in water should be enforced.
Acknowledgement
Not applicable.
Funding sources
The present research did not receive any financial support.
Ethical statement
The study was performed in accordance with the “the National Institute of Health (NIH) in the “Guide to the Care and Use of Animals in Research and Teaching published by the National Academic Press (Eighth edition, International Standard Book Number-13: 978-0-309-15400-0). The experimental protocol and procedures used in this study were approved by the College of Medicine Research Ethics Committee, Directorate of Research and Publications, College of Medicine, University of Nigeria, Enugu Campus, State, Nigeria, with protocol number 025/02/2017.
Conflicts of interest
The authors declare that there is not any conflict of interest regarding the publication of this manuscript.
Author contributions
The authors confirm contribution to the paper as follows: Study conception and design: Temidayo Daniel Adeniyi: Data collection: Temidayo Daniel Adeniyi, Akinpelu Moronkeji; Analysis and interpretation of results: Akinpelu Moronkeji, Temidayo Daniel Adeniyi, Victor Olukayode Ekundina; Draft manuscript preparation: Akinpelu Moronkeji. All authors reviewed and approved the final version of the manuscript.
Research Article: Original Paper |
Subject:
Laboratory Sciences
Received: 2022/04/12 | Accepted: 2022/08/31 | Published: 2024/01/15 | ePublished: 2024/01/15
Received: 2022/04/12 | Accepted: 2022/08/31 | Published: 2024/01/15 | ePublished: 2024/01/15
References
1. Halder J, and Islam N. Water Pollution and its Impact on the Human Health. J Environ Hum. 2015;2(1):36-46. [View at Publisher] [DOI] [Google Scholar]
2. Chen F, Lin J, Qian B, Wu Z, Huang P, Chen K, et al. Geochemical assessment and spatial analysis of heavy metals in the surface sediments in the eastern beibu gulf: A reflection on the industrial development of the South China coast. Int J Environ Res Public Health. 2018;15(3):496. [View at Publisher] [DOI] [Google Scholar]
3. Spahić MP, Sakan S, Cvetković Ž, Tančić P, Trifković J, Nikić Z, et al. Assessment of contamination, environmental risk, and origin of heavy metals in soils surrounding industrial facilities in Vojvodina, Serbia. Environ Monit Assess. 2018;190(4):208. [View at Publisher] [DOI] [Google Scholar]
4. Wang S, Xu X, Sun Y, Liu J, Li H. Heavy metal pollution in coastal areas of South China : A review. Mar Pollut Bull. 2013;76(1-2):7-15. [View at Publisher] [DOI] [Google Scholar]
5. Kim JJ, Kim YS, Kumar V. Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace Elem Med Biol. 2019;54:226-31. [View at Publisher] [DOI] [Google Scholar]
6. Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J Mol Liq. 2019; 290: 111197. [View at Publisher] [DOI] [Google Scholar]
7. Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90(1):1-37. [View at Publisher] [DOI] [Google Scholar]
8. Cao Y, Lei K, Zhang X, Xu L, Lin C, Yang Y. Contamination and ecological risks of toxic metals in the Hai River, China. Ecotoxicol Environ Saf. 2018;164:210-8. [View at Publisher] [DOI] [Google Scholar]
9. Zhang M, Sun X, Xu J. Heavy metal pollution in the East China Sea: A review. Mar Pollut Bull. 2020;159:111473. [View at Publisher] [Google Scholar] [DOI] [PMID] [View at Publisher] [DOI] [Google Scholar]
10. Akinola MO, Ekiyoyo TA. Accumulation of lead, cadmium and chromium in some plants cultivated along the bank of river Ribila at Odo-nla area of Ikorodu, Lagos state, Nigeria. J Environ Biol. 2006;27(3):597-9. [View at Publisher] [Google Scholar] [DOI] [PMID] [View at Publisher] [Google Scholar]
11. Haque MM, Niloy NM, Nayna OK, Fatema KJ, Quraishi SB, Park JH, et al. Variability of water quality and metal pollution index in the Ganges River, Bangladesh. Environ Sci Pollut Res Int. 2020;27(34):42582-99. [View at Publisher] [DOI] [Google Scholar]
12. Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):e04691. [View at Publisher] [DOI] [Google Scholar]
13. Onakpa MM, Njan AA, Kalu OC. A review of heavy metal contamination of food crops in Nigeria. Ann Glob Heal. 2018;84(3):488-94. [View at Publisher] [DOI] [Google Scholar]
14. Ahmad MK, Islam S, Rahman MS, Haque MR and, Islam MM. Heavy Metals in Water, Sediment and Some Fishes of Buriganga River, Bangladesh. Int J Environ Res. 2010;4(2):321-32. [View at Publisher] [Google Scholar]
15. National Research Council (US) Institute for Laboratory Animal Research(NAS). Guide for the Care and Use of Laboratory Animals. 8th ed. Washington DC: National Academies Press;1996. 60p. [View at Publisher] [Google Scholar]
16. Adeniyi TD, Achukwu PU, Abubakar AA. Frequency of electronics waste generated heavy metals in urban waterways. Int J Hum Cap Urb Manag. 2017;2(2):89-100. [View at Publisher] [Google Scholar]
17. Hau J, Van Hoosier G, Schapiro J, editors. Handbook of laboratory animal science: Essential principles and practices. Vol 1. US:CRC press;2002. 1-569 p. [View at Publisher] [DOI] [Google Scholar]
18. Anifowoshe AT, Oladipo S, Oyinloye AN, Opute A, Odofin E, Aiki O, et al. Cellular stress response , induction of micronuclei and DNA strand breaks in two common fish species of Rivers and Reservoirs in Ilorin , North Central, Nigeria. 2021. [View at Publisher] [DOI] [Google Scholar]
19. Orisakwe OE. Lead and cadmium in public health in Nigeria: Physicians neglect and pitfall in patient management. N Am J Med Sci. 2014;6(2):61-70. [View at Publisher] [DOI] [Google Scholar]
20. Lu Y, Song S, Wang R, Liu Z, Meng J, Sweetman AJ, et al. Impacts of soil and water pollution on food safety and health risks in China. Environ Int. 2015;77:5-15. [View at Publisher] [DOI] [Google Scholar]
21. Massányi Peter, Massányi M, Madeddu R, Stawarz R, Lukáč N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. toxics. 2020;8(4):94. [DOI]
22. Munir N, Jahangeer M, Bouyahya A, Omari N El, Ghchime R, Balahbib A, et al. Heavy metal contamination of natural foods is a serious health issue: A review. Sustain. 2022;14(1):1-20. [DOI]
23. Zhang C, Gan C, Ding L, Xiong M, Zhang A, Li P. Maternal inorganic mercury exposure and renal effects in the Wanshan mercury mining area, southwest China. Ecotoxicol Environ Saf. 2020;189:109987. [DOI]
24. Bottino C, Vázquez M, Devesa V, Laforenza U. Impaired aquaporins expression in the gastrointestinal tract of rat after mercury exposure. J Appl Toxicol. 2016;36(1):113-20. [DOI]
25. Dongre NN, Suryakar AN, Patil AJ, Ambekar JG, Rathi DB. Biochemical effects of lead exposure on systolic & diastolic blood pressure, heme biosynthesis and hematological parameters in automobile workers of North Karnataka (India). Indian J Clin Biochem. 2011;26(4):400-6. [DOI]
26. Boskabady MH, Tabatabai SA, Farkhondeh T. Inhaled Lead Affects Lung Pathology and Inflammation in Sensitized and Control Guinea Pigs. Environ Toxicol. 2016;31(4):452-60. [DOI:10.1002/tox.22058]
27. Struzyńska L, Da̧browska-Bouta B, Koza K, Sulkowski G. Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci. 2007;95(1):156-62. [DOI]
28. Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol. 2013;45(4):395-8. [DOI]
29. Deng Y, Wang M, Tian T, Lin S, Xu P, Zhou L, et al. The effect of hexavalent chromium on the incidence and mortality of human cancers: A meta-analysis based on published epidemiological cohort studies. Front Oncol. 2019;9:24. [DOI]
30. Pavesi T, Moreira JC. Mechanisms and individuality in chromium toxicity in humans. J Appl Toxicol. 2020;40(9):1183-97. [DOI]
31. Reddy SB, Charles MJ, Raju GJN, Reddy BS, Reddy TS, Lakshmi PVBR, et al. Trace elemental analysis of cancer-afflicted intestine by PIXE technique. Biol Trace Elem Res. 2004;102(1-3):265-82. [DOI]
32. Matouke M, Abdullahi KL. Assessment of heavy metals contamination and human health risk in Clarias gariepinus [ Burchell , 1822 ] collected from Jabi Lake, Abuja, Nigeria. Sci African. 2020;7:e00292. [DOI]
33. Wirth JJ, Mijal RS. Adverse Effects of Low Level Heavy Metal Exposure on Male Reproductive Function. Syst Biol Reprod Med. 2010;56(2):147-67. [DOI]
34. Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68(1):167-82. [DOI]
35. Fay MJ, Alt LAC, Ryba D, Salamah R, Peach R, Papaeliou A, et al. Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics. 2018;6(1):16. [DOI]
36. Schutte R, Nawrot TS, Richart T, Thijs L, Vanderschueren D, Kuznetsova T, et al. Bone resorption and environmental exposure to cadmium in women: A population study. Environ Health Perspect. 2008;116(6):777-83. [DOI]
37. Pan C, Liu H Da, Gong Z, Yu X, Hou X Ben, Xie DD, et al. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci Rep. 2013;3:2333. [DOI]
38. Lukáč N, Massányi P, Naď P, Slamečka J. Relationship between trace element concentrations and spermatozoa quality in rabbit semen. Slovak J Anim Sci. 2009;42(suppl):46-50.
39. Abass AT, Olayinka OS, Mutolib AO, Solomon EO, Rasheedat AA, Monsuru AA, et al. Induction of Micronuclei, Base-pair Substitution Mutation and Excision-repair Deficient by Polluted Water from Asa River in Nigeria. Ann Sci Technol. 2019;4(2):68-77. [DOI]
40. Stout MD, Herbert RA, Kissling GE, Collins BJ, Travios GS, Witt KL, et al. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ Health Perspect. 2009;117(5):716-22. [DOI]
41. Oladipo SO, Anifowoshe AT, Olafimihan TF. Assessment of Histopathological Damages In Swiss Albino Male Mice Induced By Automobile Waste Leachate. 2018;15(1):2944-9.
42. Akintunde JK, Oboh G, Akindahunsi AA. Testicular membrane lipid damage by complex mixture of leachate from municipal battery recyclingsite as indication of idiopathic male infertility in rat. Interdiscip Toxicol. 2013;6(4):192-7. [DOI]
43. Jarrar BM, Taib NT. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci. 2012;19(2):203-10. [DOI]
44. Yakubu MT, Omar SA. Impact of groundwater samples and leachates from Gbagede dumpsite, Amoyo , Kwara State , Nigeria , on testes and prostate of male Wistar rats : A biochemical and histological study. Andrologia. 2020;52(11):e13801. [DOI:10.1111/and.13801]
45. Rafati Rahimzadeh M, Kazemi S, Moghadamnia A. Cadmium toxicity and treatment: An update. Caspian J Intern Med. 2017;8(3):135-45.
46. Thorne D, Leverette R, Breheny D, Lloyd M, Mcenaney S, Whitwell J, et al. Genotoxicity evaluation of tobacco and nicotine delivery products : Part Two . In vitro micronucleus assay. Food Chem Toxicol. 2019;132:110546. [DOI]
47. De Souza Predes F, Diamante MAS, Dolder H. Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol. 2010;91(2):125-31. [DOI]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com