Volume 17, Issue 6 (Nov-Dec 2023)
mljgoums 2023, 17(6): 4-7 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Prabha T, Khan R, CN S, Priya R. Maternal and fetal outcomes among antenatal women with hypothyroid disorders in a tertiary
care center. mljgoums 2023; 17 (6) :4-7
URL: http://mlj.goums.ac.ir/article-1-1492-en.html
URL: http://mlj.goums.ac.ir/article-1-1492-en.html
1- St.Peter’s Medical College Hospital and Research Institute, Hosur, Tamil Nadu, India , drthivyahprabha@gmail.com
2- Srinivasan Medical College and Hospital, Dhanalakshmi Srinivasan University, Tiruchirapalli, Tamil Nadu, India
3- MVJ Medical College and Research Institute, Karnataka, India
4- Eastpoint Medical College Hospital and Research Institute, Karnataka, India
2- Srinivasan Medical College and Hospital, Dhanalakshmi Srinivasan University, Tiruchirapalli, Tamil Nadu, India
3- MVJ Medical College and Research Institute, Karnataka, India
4- Eastpoint Medical College Hospital and Research Institute, Karnataka, India
Full-Text [PDF 410 kb]
(791 Downloads)
| Abstract (HTML) (2767 Views)
Full-Text: (1042 Views)
Introduction
Thyroid disorders are the most prevalent cause of endocrine dysfunction among women of childbearing age (1). The development of maternal thyroid disorders during early pregnancy can significantly impact both the pregnancy outcome and fetal development. It is now well-established that not only overt thyroid dysfunction but also subclinical thyroid dysfunction can have substantial adverse effects on both pregnancy and fetal development. Adverse pregnancy outcomes associated with thyroid dysfunction include miscarriage, pregnancy-induced hypertension, preeclampsia, placental abruption, anemia, and postpartum hemorrhage. These obstetric complications contribute to an overall increase in the frequency of adverse neonatal outcomes, including preterm birth, low birth weight, increased admission to neonatal intensive care units (NICUs), and increased perinatal morbidity and mortality (2,3).
Moreover, the symptoms of hypothyroidism, such as fatigue, joint pain, muscle aches, constipation, pedal edema, dry skin, and facial puffiness are similar to symptoms experienced during pregnancy. Therefore, there is a pressing need for universal screening of all antenatal women during their first antenatal visit. Currently, there are no national guidelines in place regarding universal screening for thyroid disorders in antenatal women, and pregnant women are often screened based on risk factors.
This study aimed to diagnose thyroid disorders early in pregnancy, during the first antenatal visit, using the American Thyroid Association (ATA) guidelines. The current study differs from previous studies conducted in Bangalore that did not use the ATA guidelines (4). Additionally, some studies have used the enzyme-linked immunosorbent assay (ELISA) method to analyze thyroid hormones (5) instead of chemiluminescence. Therefore, the present study sought to identify the prevalence of both subclinical and overt thyroid disorders among antenatal women and assess the maternal and fetal outcomes of antenatal women with hypothyroid disorders.
Methods
This prospective study was conducted in the antenatal clinic at the Department of Obstetrics and Gynaecology in collaboration with the Biochemistry Department at East Point Hospital, Bangalore. The study enrolled pregnant women from the first trimester until delivery between January 2020 and January 2021. Antenatal women aged 18-40 years attending their first antenatal visit, regardless of their period of gestation, were included in the study. Known cases of hypothyroidism under treatment who attended the antenatal clinic and provided consent were also included. Written informed consent was obtained from all participants after obtaining proper ethical approval.
A structured proforma was used to collect medical histories and perform examinations on all antenatal women attending the outpatient department for the first time. Patients with multifetal gestation, metabolic disorders (such as diabetes and hypertension), pregnancy loss, or bad obstetric history were excluded from the study. Venous blood was collected from the antecubital vein, and thyrotropin, free triiodothyronine (free T3), and free thyroxine (free T4) levels were measured. Thyroid profiles were analyzed using the chemiluminescence method with the Beckman Coulter Access 2 instruments.
Antenatal women were categorized as hypothyroid based on the ATA guidelines (6). Subclinical hypothyroidism was diagnosed if the patient had normal free T4/free T3 levels and high thyrotropin, while overt hypothyroidism was diagnosed if the patient had low free T4/free T3 levels and high thyrotropin. Reference values for free T3 and free T4 were 1.7-4.2 pg/mL and 0.7-1.8 ng/dL, respectively. Trimester-specific reference values for thyrotropin, according to ATA guidelines, were as follows: first trimester, 0.1-2.5 mIU/L; second trimester, 0.2-3 mIU/L; third trimester, 0.3-3 mIU/L.
Antenatal women diagnosed with hypothyroidism were referred to obstetricians, and treatment was initiated. Thyroid function tests were repeated every 4-6 weeks during pregnancy, and drug dosages were adjusted accordingly. Hypothyroid antenatal women were followed up and observed for both maternal and fetal outcomes. Maternal outcomes, including abortion, anemia, oligohydramnios, preeclampsia, and preterm delivery, were assessed. Fetal outcomes, such as birth weight, Apgar score, hyperbilirubinemia, NICU admission, and neonatal hypo/hyperthyroidism, were also evaluated.
The sample size was calculated based on a prevalence (p) of 14% (7). All statistical analyses were performed using SPSS version 20 (SPSS Inc, Chicago, IL, USA). Continuous variables were presented as mean ± SD and analyzed using the unpaired, 2-tailed Student’s t test. P values equal to or less than 0.05 were considered statistically significant.
Results
Among the 1184 pregnant women included in the present study, 149 were found to have thyroid issues, resulting in a prevalence of thyroid disorders of 12.6%. Subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, and overt hyperthyroidism were observed in 6.9%, 3.2%, 1.8%, and 0.7% of cases, respectively.
The study revealed that a significant proportion of pregnant women with thyroid disorders fell in the age group of 25-30 years (46.3%), while fewer were in the age group of <20 years. Regarding parity among the 149 cases, 47% were primigravida, and 53% were multigravida.
The mean values of thyrotropin in cases of subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, and overt hyperthyroidism were 5.12, 8.9, 0.05, and 0.017 mIU/L, respectively.
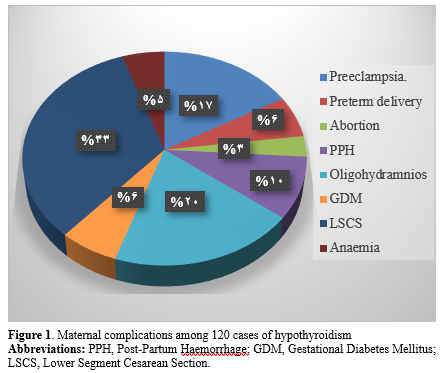
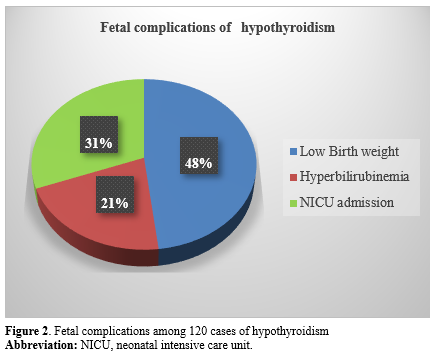
The incidence of maternal complications among the cases of hypothyroidism is described in Figure 1. The incidence of fetal complications among the hypothyroid cases included low birth weight (20.8%), hyperbilirubinemia (9.1%), and NICU admissions (13.3%), as shown in Figure 2.
A comparison between the parity of newly detected cases of hypothyroidism and known cases of hypothyroidism revealed no statistical significance between both groups. Furthermore, various complications between newly detected cases of hypothyroidism and known cases of hypothyroidism were compared, and there was no statistical significance between the 2 groups, as demonstrated in Table 1.
Discussion
The prevalence of thyroid disorders during pregnancy and the associated maternal and fetal complications can vary widely in different regions, influenced by various factors. In the present study, the prevalence of thyroid disorders was found to be 12.6%, which is consistent with the findings of studies conducted by Weiwei Wang et al (10.2%) (8), Ajamni et al (13.25%) (9), and Taghavi et al (14.6%) (10). However, the prevalence was lower in the study by Thanuja et al (5%) (11) and higher in the study by Rajput (26.5%) (12).
Regarding subclinical hypothyroidism, the prevalence in the current study was 6.5%, which is consistent with studies by Sahu et al (6.47%) (13), Weiwei Wang et al (7.2%) (8), Taghavi et al (7.4%) (10), and Sapna C Shah et al. (5.3%) (14). However, it was lower in the study by Thanuja et al (0.7%) (11) and higher in studies by Dinesh et al (13.5%) (15), Murthy et al (16.11%) (16), Singh et al (18%) (17), and Rajput et al (21.5%) (12).
The prevalence of overt hypothyroidism in the current study was 3.2%, which is consistent with studies by Taghavi et al (2.4%) (10), Bandela et al (2.87%) (18), and Ajmani et al (3%) (9). However, it was lower in studies by Weiwei Wang et al (0.3%) (8) and Dinesh (0.7%) (15) and slightly higher in studies by Sahu et al (4.58%) (13) and Singh et al (4.5%) (17).
In India, the prevalence of hypothyroidism during pregnancy is significantly higher compared with Western countries. The data from Western countries indicate that the prevalence of subclinical hypothyroidism is estimated to be 2.5%, while overt hypothyroidism is estimated to be 0.3%-0.5% during pregnancy. The prevalence of hypothyroidism during pregnancy varies significantly across different geographical regions. Due to the ongoing issue of iodine deficiency in many parts of the country, the prevalence of hypothyroidism during pregnancy shows significant variation among different states in India. Hashimoto thyroiditis is the most common cause of hypothyroidism in iodine-sufficient areas. The major causes of thyroid disorders among pregnant women are poverty, insufficient iodine supplementation, and fluorinated water. The micronutrient deficiency (such as selenium and iron deficiency) and the presence of goitrogens in diet (19) may cause hypothyroidism and goiter. In submountain areas ranging from Kashmir to northeast India, there is an observed increased prevalence of hypothyroidism (20). This can be attributed to the geochemical nature of the region, which is characterized by deficiencies in iodine and micronutrients. Geological factors, such as glaciations, high rainfall, and floods, can contribute to a decrease in the iodine content found in the soil and water sources. Serum thyrotropin and free T4 are the best tests to screen and diagnose hypothyroidism during pregnancy. The prevalence of overt or subclinical hypothyroidism in pregnancy is influenced by higher thyrotropin cutoff levels. During the early stages of gestation, the serum human chorionic gonadotropin (hCG) levels are at their peak. This hormone has a stimulating effect on the thyroid gland, leading to the lowest levels of serum thyrotropin during the first trimester of pregnancy. The American Thyroid Association recommends the use of trimester-specific reference ranges for serum thyrotropin during pregnancy. Additionally, it is recommended to consider the optimal iodine intake defined for the population. Thyroid diseases are commonly prevalent in women of childbearing age group for this reason. Untreated thyroid disorders during pregnancy have adverse effects on fetal and maternal well-being. Maintaining precise normal thyroid hormone levels in hypothyroid mothers during gestation can be challenging due to fluctuations that occur in the metabolism of T4 during pregnancy (21). The adverse pregnancy outcome may be improved by T4 replacement (22). Pregnancy causes increased thyroid gland vascularity, increased renal iodide clearance, and iodide losses to the fetus. Fluctuation in thyroxine metabolism that occurs during pregnancy may further impair maternal-fetal transfer of thyroxine despite apparently optimal thyroid status. The comparison of the incidence of complications in pregnant women having hypothyroidism is shown in Table 2.
In the current study, preeclampsia in hypothyroid patients was observed in 13.3% of cases, similar to studies by Leung et al (13.3%) (23), Ozdemir et al (14.5%) (24), Sahu et al (9.8%) (13), Taghavi et al (2.7%) (10), and Ajmani et al (22.3%) (15). The beta-adrenergic response is increased by thyroid hormones, as they increase the number of beta-adrenoceptors with an opposite action on alpha-adrenergic receptors. However, the density of alpha-1 adrenoceptors is increased, and beta-adrenoceptors are reduced on vascular beds in the hypothyroid state. Hence, the increased action of alpha-adrenoceptors mainly involves smooth muscle cell contraction, causing vasoconstriction in the blood vessel.
The significant increase in miscarriages, premature deliveries, gestational diabetes, postpartum thyroiditis, and permanent hypothyroidism (25,26) can be attributed to the presence of thyroid peroxidase antibodies (TPO-ABs) or thyroglobulin during pregnancy. In the present study, the abortion rate was 2.5%, which is consistent with the findings of Tanuja et al (1.7%) (11).
In the present study, anemia among hypothyroid patients was observed at a rate of 4.1%, which is consistent with the results of Pavanaganga et al (5.08%) (27). Additionally, the incidence of oligohydramnios among hypothyroid patients was 15.8%, while Pavanaganga et al reported a rate of 8.35%.
Regarding preterm labor, our study found it to occur in 5% of hypothyroid patients, which is consistent with the findings of Sahu et al (4.75%) (13) and Ajmani et al (5.8%) (9). In the present study, Post-partum Haemorrhage (PPH) was 7.5% in subclinical hypothyroid patients, while in the study by Mohammed et al., it was 6% (28). In our study, primary lower segment cesarean section (LSCS) was observed at a rate of 26.6% in hypothyroid patients, which is consistent with the results of Georgel et al (20%) (29). It might be commonly happening due to the fetal distress.
In the present study, gestational diabetes mellitus (GDM) was observed in 5% of hypothyroid patients, which is consistent with the results of Pavanaganga et al (6.4%) (27). The increased incidence of preeclampsia, premature delivery, postpartum depression, and hemorrhages can be attributed to the maturation process of the placental. These complications tend to occur in cases of severe hypothyroidism but have also been reported in cases of subclinical hypothyroidism (25).
In the present study, the rate of preterm delivery among hypothyroid patients was 5%, which is consistent with the findings of Sahu et al (4.7%) (13) but differs from the study conducted by Leung et al (9%) (23). Tissue factor production, triggered by aberrant vascular endothelial growth factor and inflammatory cytokine release, plays a role in promoting thrombosis.
Furthermore, shallow extra villous trophoblast (EVT) invasion may lead to placental ischemia and hemorrhage, resulting in local thrombin generation that mediates degradation. The uteroplacental interface is susceptible to both thrombosis and hemorrhage, especially in cases of structurally defective placentation. These factors might explain the observation of reduced neonatal birth weight in offspring born to mothers with inadequately controlled thyroid function, either at initial presentation or during the third trimester.
In the present study, the incidence of low birth weight in hypothyroid patients was 20.8%, which is inconsistent with the findings of Ajmani et al (12.11%) (9). Low birth weight in our study was mostly attributed to maternal pregnancy-induced hypertension (PIH), leading to intrauterine growth retardation and subsequently low birth weight. Hyperbilirubinemia in subclinical hypothyroidism was observed in 9.4% of cases in our study, which is consistent with studies by Georgel et al (8%) (29) and Leung et al (9%) (23). Additionally, in our study, NICU admission in subclinical hypothyroid patients was 14.6%, which is inconsistent with the findings of Mahadik et al (42.1%) (30).
To maintain serum thyrotropin within the trimester-specific target range, all women with overt and subclinical hypothyroidism should receive treatment during pregnancy, regardless of TPO-AB positivity with Levothyroxine (LT4). Recommendations suggest monitoring serum thyrotropin levels every 4 weeks during pregnancy to make necessary dose adjustments. The recommended therapy is oral LT4, which should be administered on an empty stomach (at least 45 minutes before consuming food, beverages, or other medications).
Immediately after delivery, the requirement of thyroxine drops. Women who were already taking thyroxine before pregnancy should return to their pre-pregnancy dosage, while those who initiated thyroxine treatment during pregnancy should reduce their dose to half of what they were taking just before delivery. For women who began thyroxine treatment during pregnancy for subclinical hypothyroidism, the medication can be discontinued after delivery. Thyroid balance should be reassessed 6 weeks postpartum, and a decision regarding continued treatment should be made accordingly.
Serum T3 and T4 levels rise within 30 minutes after delivery and remain elevated for up to 5 days due to thyrotropin elevation caused by the stress of delivery. Therefore, newborn screening should be conducted using cord blood immediately after delivery or within 5 days following delivery.
Limitations
This study has some limitations, including the omission of TPO-AB analysis in antenatal women and the lack of neonatal cord blood thyrotropin analysis, which may have implications. Future studies could consider including these variables for a more comprehensive understanding.
Conclusion
The present study revealed a high prevalence (12.6%) of thyroid disorders in pregnancy, with hypothyroidism being particularly prominent at 10.1%. Subclinical hypothyroidism was observed in 6.9% of cases, while overt hypothyroidism was observed in 3.2% of cases. Thyroxine is crucial for the brain development, growth, and lung maturation of the fetus; therefore, it is imperative to provide adequate replacement therapy to maintain thyrotropin within trimester-specific reference ranges. Thyroid testing should ideally be conducted prenatally or at the first booking to prevent miscarriages. Early and effective treatment of thyroid disorders is essential for ensuring a safe pregnancy with minimal maternal and fetal complications. Hence, universal screening for thyroid dysfunction in preconception and pregnant women is mandatory, especially in a country like India, where there is a high prevalence of undiagnosed thyroid disorders due to the asymptomatic nature of the condition in its early stages.
Acknowledgement
The authors extend their sincere appreciation to the management and all staff in the Department of Obstetrics and Gynaecology at East Point Medical College and Research Institute, Bangalore, where this study was conducted.
Funding sources
No funding sources.
Ethical statement
The study was approved by the Institutional Ethics Committee.
(EPCMSRC/ADM/IEC/2019-20/016)
Conflicts of interest
None declared.
Author contributions
Prabha T. explained the study's purpose to the patients, obtained their consent, and conducted sample analysis in the biochemistry lab.
Khan R. assisted with literature search and data analysis.
Shruthi CN. contributed to writing the manuscript.
Priya R. prescribed medications for the hypothyroid women on follow-up.
Thyroid disorders are the most prevalent cause of endocrine dysfunction among women of childbearing age (1). The development of maternal thyroid disorders during early pregnancy can significantly impact both the pregnancy outcome and fetal development. It is now well-established that not only overt thyroid dysfunction but also subclinical thyroid dysfunction can have substantial adverse effects on both pregnancy and fetal development. Adverse pregnancy outcomes associated with thyroid dysfunction include miscarriage, pregnancy-induced hypertension, preeclampsia, placental abruption, anemia, and postpartum hemorrhage. These obstetric complications contribute to an overall increase in the frequency of adverse neonatal outcomes, including preterm birth, low birth weight, increased admission to neonatal intensive care units (NICUs), and increased perinatal morbidity and mortality (2,3).
Moreover, the symptoms of hypothyroidism, such as fatigue, joint pain, muscle aches, constipation, pedal edema, dry skin, and facial puffiness are similar to symptoms experienced during pregnancy. Therefore, there is a pressing need for universal screening of all antenatal women during their first antenatal visit. Currently, there are no national guidelines in place regarding universal screening for thyroid disorders in antenatal women, and pregnant women are often screened based on risk factors.
This study aimed to diagnose thyroid disorders early in pregnancy, during the first antenatal visit, using the American Thyroid Association (ATA) guidelines. The current study differs from previous studies conducted in Bangalore that did not use the ATA guidelines (4). Additionally, some studies have used the enzyme-linked immunosorbent assay (ELISA) method to analyze thyroid hormones (5) instead of chemiluminescence. Therefore, the present study sought to identify the prevalence of both subclinical and overt thyroid disorders among antenatal women and assess the maternal and fetal outcomes of antenatal women with hypothyroid disorders.
Methods
This prospective study was conducted in the antenatal clinic at the Department of Obstetrics and Gynaecology in collaboration with the Biochemistry Department at East Point Hospital, Bangalore. The study enrolled pregnant women from the first trimester until delivery between January 2020 and January 2021. Antenatal women aged 18-40 years attending their first antenatal visit, regardless of their period of gestation, were included in the study. Known cases of hypothyroidism under treatment who attended the antenatal clinic and provided consent were also included. Written informed consent was obtained from all participants after obtaining proper ethical approval.
A structured proforma was used to collect medical histories and perform examinations on all antenatal women attending the outpatient department for the first time. Patients with multifetal gestation, metabolic disorders (such as diabetes and hypertension), pregnancy loss, or bad obstetric history were excluded from the study. Venous blood was collected from the antecubital vein, and thyrotropin, free triiodothyronine (free T3), and free thyroxine (free T4) levels were measured. Thyroid profiles were analyzed using the chemiluminescence method with the Beckman Coulter Access 2 instruments.
Antenatal women were categorized as hypothyroid based on the ATA guidelines (6). Subclinical hypothyroidism was diagnosed if the patient had normal free T4/free T3 levels and high thyrotropin, while overt hypothyroidism was diagnosed if the patient had low free T4/free T3 levels and high thyrotropin. Reference values for free T3 and free T4 were 1.7-4.2 pg/mL and 0.7-1.8 ng/dL, respectively. Trimester-specific reference values for thyrotropin, according to ATA guidelines, were as follows: first trimester, 0.1-2.5 mIU/L; second trimester, 0.2-3 mIU/L; third trimester, 0.3-3 mIU/L.
Antenatal women diagnosed with hypothyroidism were referred to obstetricians, and treatment was initiated. Thyroid function tests were repeated every 4-6 weeks during pregnancy, and drug dosages were adjusted accordingly. Hypothyroid antenatal women were followed up and observed for both maternal and fetal outcomes. Maternal outcomes, including abortion, anemia, oligohydramnios, preeclampsia, and preterm delivery, were assessed. Fetal outcomes, such as birth weight, Apgar score, hyperbilirubinemia, NICU admission, and neonatal hypo/hyperthyroidism, were also evaluated.
The sample size was calculated based on a prevalence (p) of 14% (7). All statistical analyses were performed using SPSS version 20 (SPSS Inc, Chicago, IL, USA). Continuous variables were presented as mean ± SD and analyzed using the unpaired, 2-tailed Student’s t test. P values equal to or less than 0.05 were considered statistically significant.
Results
Among the 1184 pregnant women included in the present study, 149 were found to have thyroid issues, resulting in a prevalence of thyroid disorders of 12.6%. Subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, and overt hyperthyroidism were observed in 6.9%, 3.2%, 1.8%, and 0.7% of cases, respectively.
The study revealed that a significant proportion of pregnant women with thyroid disorders fell in the age group of 25-30 years (46.3%), while fewer were in the age group of <20 years. Regarding parity among the 149 cases, 47% were primigravida, and 53% were multigravida.
The mean values of thyrotropin in cases of subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, and overt hyperthyroidism were 5.12, 8.9, 0.05, and 0.017 mIU/L, respectively.
The incidence of maternal complications among the cases of hypothyroidism is described in Figure 1. The incidence of fetal complications among the hypothyroid cases included low birth weight (20.8%), hyperbilirubinemia (9.1%), and NICU admissions (13.3%), as shown in Figure 2.


A comparison between the parity of newly detected cases of hypothyroidism and known cases of hypothyroidism revealed no statistical significance between both groups. Furthermore, various complications between newly detected cases of hypothyroidism and known cases of hypothyroidism were compared, and there was no statistical significance between the 2 groups, as demonstrated in Table 1.
Table 1. Various outcomes in newly detected and known cases of hypothyroidism (n = 120) |
Discussion
The prevalence of thyroid disorders during pregnancy and the associated maternal and fetal complications can vary widely in different regions, influenced by various factors. In the present study, the prevalence of thyroid disorders was found to be 12.6%, which is consistent with the findings of studies conducted by Weiwei Wang et al (10.2%) (8), Ajamni et al (13.25%) (9), and Taghavi et al (14.6%) (10). However, the prevalence was lower in the study by Thanuja et al (5%) (11) and higher in the study by Rajput (26.5%) (12).
Regarding subclinical hypothyroidism, the prevalence in the current study was 6.5%, which is consistent with studies by Sahu et al (6.47%) (13), Weiwei Wang et al (7.2%) (8), Taghavi et al (7.4%) (10), and Sapna C Shah et al. (5.3%) (14). However, it was lower in the study by Thanuja et al (0.7%) (11) and higher in studies by Dinesh et al (13.5%) (15), Murthy et al (16.11%) (16), Singh et al (18%) (17), and Rajput et al (21.5%) (12).
The prevalence of overt hypothyroidism in the current study was 3.2%, which is consistent with studies by Taghavi et al (2.4%) (10), Bandela et al (2.87%) (18), and Ajmani et al (3%) (9). However, it was lower in studies by Weiwei Wang et al (0.3%) (8) and Dinesh (0.7%) (15) and slightly higher in studies by Sahu et al (4.58%) (13) and Singh et al (4.5%) (17).
In India, the prevalence of hypothyroidism during pregnancy is significantly higher compared with Western countries. The data from Western countries indicate that the prevalence of subclinical hypothyroidism is estimated to be 2.5%, while overt hypothyroidism is estimated to be 0.3%-0.5% during pregnancy. The prevalence of hypothyroidism during pregnancy varies significantly across different geographical regions. Due to the ongoing issue of iodine deficiency in many parts of the country, the prevalence of hypothyroidism during pregnancy shows significant variation among different states in India. Hashimoto thyroiditis is the most common cause of hypothyroidism in iodine-sufficient areas. The major causes of thyroid disorders among pregnant women are poverty, insufficient iodine supplementation, and fluorinated water. The micronutrient deficiency (such as selenium and iron deficiency) and the presence of goitrogens in diet (19) may cause hypothyroidism and goiter. In submountain areas ranging from Kashmir to northeast India, there is an observed increased prevalence of hypothyroidism (20). This can be attributed to the geochemical nature of the region, which is characterized by deficiencies in iodine and micronutrients. Geological factors, such as glaciations, high rainfall, and floods, can contribute to a decrease in the iodine content found in the soil and water sources. Serum thyrotropin and free T4 are the best tests to screen and diagnose hypothyroidism during pregnancy. The prevalence of overt or subclinical hypothyroidism in pregnancy is influenced by higher thyrotropin cutoff levels. During the early stages of gestation, the serum human chorionic gonadotropin (hCG) levels are at their peak. This hormone has a stimulating effect on the thyroid gland, leading to the lowest levels of serum thyrotropin during the first trimester of pregnancy. The American Thyroid Association recommends the use of trimester-specific reference ranges for serum thyrotropin during pregnancy. Additionally, it is recommended to consider the optimal iodine intake defined for the population. Thyroid diseases are commonly prevalent in women of childbearing age group for this reason. Untreated thyroid disorders during pregnancy have adverse effects on fetal and maternal well-being. Maintaining precise normal thyroid hormone levels in hypothyroid mothers during gestation can be challenging due to fluctuations that occur in the metabolism of T4 during pregnancy (21). The adverse pregnancy outcome may be improved by T4 replacement (22). Pregnancy causes increased thyroid gland vascularity, increased renal iodide clearance, and iodide losses to the fetus. Fluctuation in thyroxine metabolism that occurs during pregnancy may further impair maternal-fetal transfer of thyroxine despite apparently optimal thyroid status. The comparison of the incidence of complications in pregnant women having hypothyroidism is shown in Table 2.
In the current study, preeclampsia in hypothyroid patients was observed in 13.3% of cases, similar to studies by Leung et al (13.3%) (23), Ozdemir et al (14.5%) (24), Sahu et al (9.8%) (13), Taghavi et al (2.7%) (10), and Ajmani et al (22.3%) (15). The beta-adrenergic response is increased by thyroid hormones, as they increase the number of beta-adrenoceptors with an opposite action on alpha-adrenergic receptors. However, the density of alpha-1 adrenoceptors is increased, and beta-adrenoceptors are reduced on vascular beds in the hypothyroid state. Hence, the increased action of alpha-adrenoceptors mainly involves smooth muscle cell contraction, causing vasoconstriction in the blood vessel.
The significant increase in miscarriages, premature deliveries, gestational diabetes, postpartum thyroiditis, and permanent hypothyroidism (25,26) can be attributed to the presence of thyroid peroxidase antibodies (TPO-ABs) or thyroglobulin during pregnancy. In the present study, the abortion rate was 2.5%, which is consistent with the findings of Tanuja et al (1.7%) (11).
In the present study, anemia among hypothyroid patients was observed at a rate of 4.1%, which is consistent with the results of Pavanaganga et al (5.08%) (27). Additionally, the incidence of oligohydramnios among hypothyroid patients was 15.8%, while Pavanaganga et al reported a rate of 8.35%.
Regarding preterm labor, our study found it to occur in 5% of hypothyroid patients, which is consistent with the findings of Sahu et al (4.75%) (13) and Ajmani et al (5.8%) (9). In the present study, Post-partum Haemorrhage (PPH) was 7.5% in subclinical hypothyroid patients, while in the study by Mohammed et al., it was 6% (28). In our study, primary lower segment cesarean section (LSCS) was observed at a rate of 26.6% in hypothyroid patients, which is consistent with the results of Georgel et al (20%) (29). It might be commonly happening due to the fetal distress.
Table 2. Comparison of the incidence of complications in pregnant women having hypothyroidism |
In the present study, the rate of preterm delivery among hypothyroid patients was 5%, which is consistent with the findings of Sahu et al (4.7%) (13) but differs from the study conducted by Leung et al (9%) (23). Tissue factor production, triggered by aberrant vascular endothelial growth factor and inflammatory cytokine release, plays a role in promoting thrombosis.
Furthermore, shallow extra villous trophoblast (EVT) invasion may lead to placental ischemia and hemorrhage, resulting in local thrombin generation that mediates degradation. The uteroplacental interface is susceptible to both thrombosis and hemorrhage, especially in cases of structurally defective placentation. These factors might explain the observation of reduced neonatal birth weight in offspring born to mothers with inadequately controlled thyroid function, either at initial presentation or during the third trimester.
In the present study, the incidence of low birth weight in hypothyroid patients was 20.8%, which is inconsistent with the findings of Ajmani et al (12.11%) (9). Low birth weight in our study was mostly attributed to maternal pregnancy-induced hypertension (PIH), leading to intrauterine growth retardation and subsequently low birth weight. Hyperbilirubinemia in subclinical hypothyroidism was observed in 9.4% of cases in our study, which is consistent with studies by Georgel et al (8%) (29) and Leung et al (9%) (23). Additionally, in our study, NICU admission in subclinical hypothyroid patients was 14.6%, which is inconsistent with the findings of Mahadik et al (42.1%) (30).
To maintain serum thyrotropin within the trimester-specific target range, all women with overt and subclinical hypothyroidism should receive treatment during pregnancy, regardless of TPO-AB positivity with Levothyroxine (LT4). Recommendations suggest monitoring serum thyrotropin levels every 4 weeks during pregnancy to make necessary dose adjustments. The recommended therapy is oral LT4, which should be administered on an empty stomach (at least 45 minutes before consuming food, beverages, or other medications).
Immediately after delivery, the requirement of thyroxine drops. Women who were already taking thyroxine before pregnancy should return to their pre-pregnancy dosage, while those who initiated thyroxine treatment during pregnancy should reduce their dose to half of what they were taking just before delivery. For women who began thyroxine treatment during pregnancy for subclinical hypothyroidism, the medication can be discontinued after delivery. Thyroid balance should be reassessed 6 weeks postpartum, and a decision regarding continued treatment should be made accordingly.
Serum T3 and T4 levels rise within 30 minutes after delivery and remain elevated for up to 5 days due to thyrotropin elevation caused by the stress of delivery. Therefore, newborn screening should be conducted using cord blood immediately after delivery or within 5 days following delivery.
Limitations
This study has some limitations, including the omission of TPO-AB analysis in antenatal women and the lack of neonatal cord blood thyrotropin analysis, which may have implications. Future studies could consider including these variables for a more comprehensive understanding.
Conclusion
The present study revealed a high prevalence (12.6%) of thyroid disorders in pregnancy, with hypothyroidism being particularly prominent at 10.1%. Subclinical hypothyroidism was observed in 6.9% of cases, while overt hypothyroidism was observed in 3.2% of cases. Thyroxine is crucial for the brain development, growth, and lung maturation of the fetus; therefore, it is imperative to provide adequate replacement therapy to maintain thyrotropin within trimester-specific reference ranges. Thyroid testing should ideally be conducted prenatally or at the first booking to prevent miscarriages. Early and effective treatment of thyroid disorders is essential for ensuring a safe pregnancy with minimal maternal and fetal complications. Hence, universal screening for thyroid dysfunction in preconception and pregnant women is mandatory, especially in a country like India, where there is a high prevalence of undiagnosed thyroid disorders due to the asymptomatic nature of the condition in its early stages.
Acknowledgement
The authors extend their sincere appreciation to the management and all staff in the Department of Obstetrics and Gynaecology at East Point Medical College and Research Institute, Bangalore, where this study was conducted.
Funding sources
No funding sources.
Ethical statement
The study was approved by the Institutional Ethics Committee.
(EPCMSRC/ADM/IEC/2019-20/016)
Conflicts of interest
None declared.
Author contributions
Prabha T. explained the study's purpose to the patients, obtained their consent, and conducted sample analysis in the biochemistry lab.
Khan R. assisted with literature search and data analysis.
Shruthi CN. contributed to writing the manuscript.
Priya R. prescribed medications for the hypothyroid women on follow-up.
Research Article: Research Article |
Subject:
Biochemistry
Received: 2022/03/4 | Accepted: 2022/12/14 | Published: 2024/02/26 | ePublished: 2024/02/26
Received: 2022/03/4 | Accepted: 2022/12/14 | Published: 2024/02/26 | ePublished: 2024/02/26
References
1. Gupta K. Thyroid disorders and pregnancy. FOGSI FOCUS- Medical Disorders in pregnancy. 2009;10: 59-66.
2. Wilson GR, Curry RW. Subclinical Thyroid Disease. Am Fam physician 2005;72(8):1517-24. [View at Publisher] [PMID] [Google Scholar]
3. Reid SM, Middleton P, Cossich MC, Crowther CA. Interventions for clinical and subclinical hypothyroidism in pregnancy. Cochrane Database Syst Rev. 2010;(7):CD007752. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Dhanwal DK, Bajaj S, Rajput R, Subramaniam KAV, Chowdhury S, Bhandari R, et al. Prevalence of hypothyroidism in pregnancy: An epidemiological study from 11 cities in 9 states of India. Indian J Endocrinol Metab. 2016;20(3):387-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Nataraj HG, Sreelatha S, Ramya S. Prevalence of Sub Clinical Hypothyroidism In First Trimester Of Pregnancy. J of Evidence Based Med Hlthcare. 2015;2(15):2292-5. [View at Publisher] [DOI] [Google Scholar]
6. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27(3):315-89. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Korde VR, Barse SP, Barla JS. Prevalence of thyroid dysfunctions in pregnant women: a prospective study in a tertiary care hospital in Maharashtra, India. Int J Reprod Contracept Obstet Gynecol. 2018;7(8):3211-5. [View at Publisher] [DOI] [Google Scholar]
8. Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: The benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011;164(2):263-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Ajmani SN, Aggarwal D, Bhatia P, Sharma M, Sarabhai V, Paul M. Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India. 2014;64(2):105-10. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Taghavi M, Saghafi N, Shirin S. Outcome of Thyroid Dysfunction in Pregnancy in Mashhad, Iran. Int J Endocrinol Metab. 2009;7(2):82-5. [View at Publisher] [Google Scholar]
11. Thanuja PM, Rajgopal K, Sadiqunnisa. Thyroid dysfunction in pregnancy and its maternal outcome. J Dent Med Sci. 2014;13(1):11-5. [View at Publisher] [DOI] [Google Scholar]
12. Rajesh R, Vasudha G, Smiti N, Meena R, Shashi S. Prevalence of thyroid dysfunction among women during the first trimester of pregnancy at a tertiary care hospital in Haryana. Indian J Endocrinol Metab. 2015;19(3):416-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Obstet Gynaecol. 2010; 281(2):215-220. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. C Shah S, R Shah C. Thyroid disorders in pregnancy - a comparative study. Indian journal of fundamental and applied life sciences. 2015;5(1):7-14. [View at Publisher] [Google Scholar]
15. Dhanwal DK, Prasad S, Agarwal AK, Dixit V, Banerjee AK. High prevalence of subclinical hypothyroidism during first trimester of pregnancy in north India. Indian J Endocrinol Metab. 2013;17(2):281-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Murty NVR, Uma B, Rao JM, Sampurna K, Vasantha K, Vijayalakshmi G. High prevalence of subclinical hypothyroidism in pregnant women in South India. Int J Reprod. Contracept Obstet Gynecol. 2015;4(2):453-6. [View at Publisher] [DOI] [Google Scholar]
17. Singh KP, Singh HA, Kamei H, Madhuri DL. Prevalence of hypothyroidism among pregnant women in the sub mountain state of Manipur. Int J Sci Study. 2015;3(5):143-6. [View at Publisher] [DOI] [Google Scholar]
18. Pandit VB, Havilah P, Hindhumathi M, Durga PK. Antenatal thyroid dysfunction in Rayalaseema region: A preliminary cross sectional study based on circulating serum thyrotropin levels. Int J Appl Biol Pharm. 2013;4(4):74-8. [View at Publisher] [Google Scholar]
19. Marwaha RK, Tandon N, Gupta N, Karak AK, Verma K, Kochupillai N. Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol. 2003;59(6):672-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Gupta MC, Mahajan BK. Text Book of Preventive and Social Medicine. 4th ed. New Delhi: Jaypee Brothers Medical;2003. p.4182-421. [View at Publisher] [Google Scholar]
21. Park CE. Evaluation of Pregnancy and Thyroid Function. Korean J Clin Lab Sci. 2018;50(1):1-10. [View at Publisher] [DOI] [Google Scholar]
22. Jameson J, Mandel SJ, Weetman AP. Disorders of the Thyroid Gland. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, et al. Harrison's Principles of Internal Medicine. 16th ed. McGraw Hill;2005. p.2104-26. [View at Publisher] [Google Scholar]
23. Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993;81(3):349-53. [View at Publisher] [PMID] [Google Scholar]
24. Ozdemir H, Akman I, Coskun S, Demirel U, Turan S, Bereket A, et al. Maternal thyroid dysfunction and Neonatal thyroid problems. Int J Endocrinol. 2013;2013:987843. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Banerjee S. Thyroid disorders in pregnancy. J Assoc Physicians India. 2011;59:32-4. [View at Publisher] [PMID] [Google Scholar]
27. Pavanaganga A, Rekha BR, Sailakshmi MPA, Nagarathnamma R. Observational study of subclinical hypothyroidism in pregnancy. Indian J Obstet Gynaecol Res. 2015:2(4):255-60. [View at Publisher] [DOI] [Google Scholar]
28. Mohammed MZ, Chandrashekar K. Clinical study of pregnancy with hypothyroidism and its outcome in Tertiary care hospital. J Evol Med Dental Sci. 2015;4(94):15927-9. [View at Publisher] [DOI] [Google Scholar]
29. George M, George SM, Thankachi VMJ. Hypothyroid in pregnancy screen or not. J Evol Med Dent Sci. 2015;4(29):4973-8. [View at Publisher] [DOI] [Google Scholar]
30. Mahadik K, Choudhary P, Roy PK. Study of thyroid function in pregnancy, its feto-maternal outcome; a prospective observational study. BMC Pregnancy Childbirth. 2020;20(1):769. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92(8Suppl):S1-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Sreelatha S, Nadagoudar S, Devi AL. The study of maternal and fetal outcome in pregnant women with thyroid disorders. Int J Reprod Contracept Obstet Gynecol. 2017;6(8):3507-13. [View at Publisher] [DOI] [Google Scholar]
33. Hareesh MV, Bijju A, Steephan S, Mathew A. The profile of infants born to mothers with subclincical hypothyrodism in tertiary care centre. IOSR-JDMS. 2015;14(11):42-6. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








