Volume 18, Issue 4 (Jul-Aug 2024)
mljgoums 2024, 18(4): 32-35 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shapoori A, Asgharpour H, Farzanegi P, Aghaei Bahman Beglou N. The effects of swimming, cell therapy, and laser therapy on the expression of dynamin-related protein 1, muscle-specific ring finger protein 1, and mitofusin 2 genes in the testes of azoospermic rats. mljgoums 2024; 18 (4) :32-35
URL: http://mlj.goums.ac.ir/article-1-1473-en.html
URL: http://mlj.goums.ac.ir/article-1-1473-en.html
1- Department of Physical Education and Sports Sciences, Aliabad Katoul Branch, Islamic Azad University, Aliabad katoul, Iran
2- Department of Physical Education and Sports Sciences, Aliabad Katoul Branch, Islamic Azad University, Aliabad katoul, Iran ,Dr.habibasgharpour@aliabadiau.ac.ir
3- Department of Physical Education and Sports Sciences, Sari Branch, Islamic Azad University, Sari, Iran
2- Department of Physical Education and Sports Sciences, Aliabad Katoul Branch, Islamic Azad University, Aliabad katoul, Iran ,
3- Department of Physical Education and Sports Sciences, Sari Branch, Islamic Azad University, Sari, Iran
Full-Text [PDF 361 kb]
(477 Downloads)
| Abstract (HTML) (1759 Views)
Discussion
The induction of the azoospermia model caused a significant decrease in testicular tissue Mfn2 gene, which was enhanced by exercise, laser, cell, and combination methods in testicular tissue, which was only significant in the exercise + laser group. Regarding Drp1 gene expression in rats’ testicular tissues, the induction of the azoospermia model increased the expression of this gene, but the treatment methods used decreased the expression of this gene, which was not significant in any of the groups. Also, the induction of the azoospermia model significantly increased the Murf1 gene expression in the testicular tissue, but the treatment methods decreased the expression of this gene, which was significant in the exercise, cell, exercise + laser, exercise + cell, and cell + laser groups.
Muzaffar et al. showed a significant improvement in spermatogenesis and testicular tissue indices in samples treated with culture medium obtained from bone marrow mesenchymal stem cells, compared to the induced azoospermic group (6). Other studies in animals, such as hamsters and mice, have demonstrated a significant improvement in spermatogenesis due to stem cell therapy (10,11). On the other hand, studies have shown that many cellular pathways are regulated by cell redox status. Modulation of cellular redox status by nuclear factor kappa B (NF-κB) transcription factor and phospholipase A2 increases DNA synthesis. Therefore, low-power laser accelerates the rhythm of mitotic and meiotic divisions in spermatogenesis and increases the number of germ cells, especially primary spermatocytes (7). In addition to affecting oxidative phosphorylation in mitochondria and increasing ATP production, low-power laser beams increase microcirculation (Microscopic circulation). Laser causes vasodilation by releasing chemicals, such as histamine, which enhances cellular metabolism along with increasing oxidative phosphorylation of cells (1, 7). Increased microcirculation in the testes improves the metabolic status, which, after laser irradiation, justifies the important biological stimulatory effect on spermatogenesis (12).
Also, based on the results of previous research, exercise has positive effects on mitochondrial health (13). Zeng et al. showed a significant increase in Mfn2 protein related to skeletal muscle mitochondrial fusion in elderly mice after exercise interventions (14). Also, comparing the mitochondrial fusion stress response to exercise activity, studies have shown that the expression of genes associated with mitochondrial cleavage significantly increases after prolonged exercise (15). According to Fealy et al.’s study, exercise reduces the activity of Drp1 protein in skeletal muscle (16). One of the properties of Drp1 is apoptosis in mitochondria, so exercise can protect tissues against oxidative stress and reduce Drp1 levels by reducing fat peroxides and activating antioxidant defenses. Decreased Drp1 reduces cleavage and apoptosis in cells and improves apoptotic-dependent markers (17). The reduction of Drp1 is essential for the development and maintenance of proper mitochondrial function, and it improves cell survival by reducing oxidative stress (18).
The ubiquitin-proteasome pathway is applied to the entire cell and involves many substrates and reaction processes in vivo, including the ubiquitination of substrate proteins and the degradation of ubiquitinated proteins. Ubiquitin E3 ligases, such as atrogin-1 and Murf1, are essential for protein ubiquitination (19). In Zheng’s research, exercise significantly reduced Murf1 gene expression in the skeletal muscle of older rats, which may subsequently inhibit the process of protein ubiquitination (14). The regulation of Murf1, including the NF-κB and forkhead box O3a (FoxO3a) signaling pathways, has a cumulative effect on skeletal muscle atrophy, with NF-κB being one of the most important transcription factors for the regulation of Murf1 involved in senile skeletal muscle atrophy (20). Exercise can reduce the expression of Murf1 proteins mediated by inhibiting oxidative stress (21,22).
In this study, each of the treatment method was effective in mitochondrial dynamics, but in each of the combined methods, a better result was obtained, and they had a synergistic effect, and this synergy was more in the laser + exercise group than in other combined methods.
Conclusion
According to the research results, it is possible that the combination of swimming exercise with cell therapy and laser therapy by changing some of the genes involved in the mitochondrial dynamics of the testicular tissue of experimental rats with azoospermia may cause the rats to become fertile, but a definite opinion needs more research in this regard.
Acknowledgement
This research was extracted from a doctoral dissertation at Islamic Azad University, Aliabad Katoul Branch. The authors hereby express their gratitude and appreciation to this academic center.
Funding sources
This article received no funding and was conducted at the personal expense of the authors.
Ethical statement
All steps of the present research have been approved by the Research Ethics Committee of Islamic Azad University, Sari Branch (IR.IAU.SARI.REC.1398.149).
Conflicts of interest
No conflict of interest.
Author contributions
All authors contributed equally to the writing of this article.
Full-Text: (283 Views)
Introduction
One of the causes of infertility is azoospermia, which refers to the lack of sperm in each ejaculation and affects about 1% of men in the total population (1). Non-obstructive azoospermia is a condition in which no sperm is observed in ejaculation and is related to intra-testicular disorders resulting in impaired spermatogenesis, while in obstructive azoospermia, spermatogenesis is normal, and the defect is related to ejaculatory duct obstruction (1).
Mitochondria are cellular organs that exist in dynamic networks. Mitochondria make up 30-35% of the cell volume, and adenosine triphosphate (ATP) production being their primary function. Mitochondrial dynamics, especially fusion and fission, are essential processes in mitochondrial homeostasis (2). Mitochondrial dynamics are regulated by several different guanosine triphosphatases (GTPases) (1). Mitofusin 2 (Mfn2), mitofusin 1 (Mfn1), and optic atrophy 1 (OPA1) lead to mitochondrial fusion in the outer and inner membranes. Dynamin-related protein 1 (Drp1), on the other hand, is a cytoplasmic protein that, upon activation, translocates to the mitochondrial membrane and promotes cleavage with the interaction of cleft protein (mitochondrial fission 1 protein [FIS1]) (3,4). Since fusion mediators regulate mitochondrial metabolism in addition to mitochondrial and endoplasmic reticulum binding, their regulatory decline is usually associated with a decrease in mitochondrial oxidative capacity (4).
One of the methods of treating azoospermia is the use of mesenchymal stem cells (1). Bone marrow mesenchymal stem cells have a high ability to differentiate into different cell lines (5). The medium used in bone marrow mesenchymal stem cell cultures can induce the return of spermatogenesis in induced azoospermic rats (6).
One of the most important applications of lasers in medical science is the use of low-power laser therapy (LLLT) or laser biostimulation to treat patients. LLLT is a treatment method that uses light radiation with low intensity and causes a change in the permeability of the cell membrane, followed by the production of mRNA and cell division. It can affect the rate of proliferation and colonization of spermatogonial stem cells (1,7). Numerous studies have also supported exercise as a strategy to reverse the effects of mitochondrial disorders and to prevent or treat disease (1). Physical activity can increase the amount of sex hormones, sperm production, and fertility, and also prevent the testicles from shrinking and increase the amount of semen (8).
Among the aerobic exercises, low-intensity swimming aerobic exercise is one of the exercises that is safe and usable in various physiological conditions and is used in most physiological, biochemical, and molecular reaction studies due to its intolerance to aquatic compared to non-aquatic sports. On the other hand, no study has been found to investigate the simultaneous effects of swimming, cell therapy, and laser therapy on azoospermic rats. Therefore, this study aims to investigate whether regular aerobic exercise, laser therapy, and cell therapy affect the expression of genes involved in mitochondrial dynamics of testicular tissue in azoospermic rats.
Methods
In the present experimental research, 40 male Wistar rats (6-8-week-old) were purchased from the Center for Research and Reproduction of Laboratory Animals in Tehran after transferring the subjects to the laboratory and after one week. Adaptation to the new environment was maintained in groups of 5 in transparent polycarbonate cages in an environment with a mean temperature of 22 ± 1.4 °C, humidity of 55%, and a dark cycle of 12:12 hours. The care of the animals was carried out in accordance with the guidelines of the International Institute of Health and the protocols of this study, in accordance with the principles of the Helsinki Declaration, and the rules of medical ethics. Also, during the research, the animals were fed with a food pack (Made by Behparvar Karaj Company) at the rate of 10 g per 100 g body weight per day (According to weekly weight gain) and had free access to drinking water through bottles.
In order to create an azoospermia model, busulfan at a dose of 40 mg /kg body weight was then injected intraperitoneally into each rat (the waiting time for creating the model was 30 days) (9).
One month after model induction, rats were grouped as follows:1) Healthy control group (Maintained for 8 weeks); 2) sham group; 3) patient group (Remained for 8 weeks until the end of the study; 4) patient + laser group (One month after the model was developed, a low-power laser with a wavelength of 632.8 nm, a power of 10 mW, and an energy of 3 joules was applied to the testes of azoospermic rats in three repetitions throughout the study period at weekly intervals, and rats were kept for 8 weeks until the end of the study; 5) patient + exercise group (One month after azoospermia, the rats swam with low intensity for 30 minutes a day, 5 days a week, for 8 weeks; 6) patient + cell group (One month after azoospermia, once stem cells were transplanted in the vas deferens at the rate of one million cells per rat in the right testicle, and the rats were kept for 8 weeks until the end of the study); 7) patient + exercise + laser group (One month after azoospermia, low-power laser with a wavelength of 632.8 nm, a power of 10 mW, and an energy of 3 joules was applied in three repetitions throughout the study period with an interval of once a week. Then, after one week, rats swam for 30 minutes a day, for 5 days a week, for 8 weeks); and 8) patient + cell + exercise group (One month after azoospermia, once stem cells were transplanted in the vas deferens at the rate of one million cells per rat. Then, after one week of transplantation cells, rats swam for 30 minutes a day, for 5 days a week, for 8 weeks).
Before starting the main protocol, the rats in the exercise groups were placed in a water pool for 20 minutes, each time for one week (5 days), in order to being familiarized with the water, reduce the stress of swimming, and adapt to the exercise conditions. Then, they swam 5 days a week until the end of the research period in a water tank with dimensions of 50 x 50 x 100 cm with a temperature of 30-32℃ for 8 weeks. The duration of exercise in water was 30 minutes daily until the end of the exercise period (1).
Sampling of rats’ testicular tissues was performed under very similar conditions and in baseline conditions (Two days after the end of the exercise period). In order to eliminate the acute effects of exercise, the animals were sampled 48 hours following the last swimming exercise program. For this purpose, the animals were first anesthetized using the peritoneal injection of ketamine (50-30 mg / kg) and xylazine (3-5 mg / kg) and then killed. After killing the transplanted tissues, they were evaluated for genetic studies.
To investigate the expression of the studied genes in each group, tissue analysis was performed using the real-time polymerase chain reaction (RT-PCR) technique. First, primer design was performed, and then total RNA was extracted from tissues and converted to cDNA. The cDNA was then amplified by PCR and examined for the expression of the mentioned genes. For molecular studies on the gene expression level, RNA was extracted from tissues in all study groups according to the manufacturer’s protocol (Kiagen, Germany). After extracting RNA with high purity and concentration from all samples, cDNA was synthesized according to the manufacturer’s protocol (Fermentas, USA), and then the synthesized cDNA was used for a reverse transcription reaction. For the real-time quantitative PCR (RT-qPCR) technique, first, the RNA of all cells was extracted according to the synagen protocol using chiazol solution and exposed to deoxyribonuclease I (DNase I) (Fermentas) to ensure contamination with genomic DNA. Then, the quality of the extracted RNAs was evaluated by a spectrophotometric device (DPI-1, Kiagen). To prepare a single-stranded cDNA from Oligodt primer (MWG-Biotech, Germany) and reverse transcription enzyme (Fermentas) was performed according to the relevant protocol. Each PCR reaction was performed using PCR master mix Applied Biosystems and SYBER Green in the device ABI Step One (Applied Biosystems, Sequences Detection Systems Focter City, CA.) according to the manufacturer's protocol. Forty cycles were considered for each RT-PCR cycle, and the temperatures of each cycle were set, including 94 °C for 20 seconds, 60-58 °C for 30 seconds, and 72 °C for 30 seconds. A melting diagram was performed to evaluate the accuracy of PCR reactions, and it was evaluated specifically for each gene and in each reaction, with a negative control diagram to check for contamination in each reaction.
To analyze the findings of this study, the Kolmogorov-Smirnov test, Fisher’s one-way analysis of variance (ANOVA), and Tukey’s test were used for comparison between different groups. All calculations were performed using SPSS statistical software version 22 at a significant level of P ≥ 0.05.
Results
Examining the mean and standard deviation of Mfn2 levels in different research groups shows a significant difference in Mfn2 between research groups (F=4.197; P=0.002). The highest Mfn2 index levels belonged to the laser-exercise group, and the lowest levels belonged to the patient group. The results of the one-way ANOVA on the levels of Mfn2 in different groups indicate a significant difference in the Mfn2 levels between different groups. Induction of the experimental model of azoospermia decreased the Mfn2 gene expression in the testicular tissue compared to the healthy control group. The simultaneous implementation of laser + exercise significantly increased the expression of this gene compared to the patient group (P=0.004) (Table 1; Figure 1).
Examining the mean and standard deviation of Drp1 levels in different research groups shows no significant differences in Drp1 between research groups (F=2.577; P=0.057). The highest levels of the Drp1 index belonged to the sham group, and the lowest belonged to the healthy control group. The results of the one-way ANOVA in Drp1 levels between different research groups show no significant difference in the Drp1 levels between different groups (Table 2; Figure 2).
One of the causes of infertility is azoospermia, which refers to the lack of sperm in each ejaculation and affects about 1% of men in the total population (1). Non-obstructive azoospermia is a condition in which no sperm is observed in ejaculation and is related to intra-testicular disorders resulting in impaired spermatogenesis, while in obstructive azoospermia, spermatogenesis is normal, and the defect is related to ejaculatory duct obstruction (1).
Mitochondria are cellular organs that exist in dynamic networks. Mitochondria make up 30-35% of the cell volume, and adenosine triphosphate (ATP) production being their primary function. Mitochondrial dynamics, especially fusion and fission, are essential processes in mitochondrial homeostasis (2). Mitochondrial dynamics are regulated by several different guanosine triphosphatases (GTPases) (1). Mitofusin 2 (Mfn2), mitofusin 1 (Mfn1), and optic atrophy 1 (OPA1) lead to mitochondrial fusion in the outer and inner membranes. Dynamin-related protein 1 (Drp1), on the other hand, is a cytoplasmic protein that, upon activation, translocates to the mitochondrial membrane and promotes cleavage with the interaction of cleft protein (mitochondrial fission 1 protein [FIS1]) (3,4). Since fusion mediators regulate mitochondrial metabolism in addition to mitochondrial and endoplasmic reticulum binding, their regulatory decline is usually associated with a decrease in mitochondrial oxidative capacity (4).
One of the methods of treating azoospermia is the use of mesenchymal stem cells (1). Bone marrow mesenchymal stem cells have a high ability to differentiate into different cell lines (5). The medium used in bone marrow mesenchymal stem cell cultures can induce the return of spermatogenesis in induced azoospermic rats (6).
One of the most important applications of lasers in medical science is the use of low-power laser therapy (LLLT) or laser biostimulation to treat patients. LLLT is a treatment method that uses light radiation with low intensity and causes a change in the permeability of the cell membrane, followed by the production of mRNA and cell division. It can affect the rate of proliferation and colonization of spermatogonial stem cells (1,7). Numerous studies have also supported exercise as a strategy to reverse the effects of mitochondrial disorders and to prevent or treat disease (1). Physical activity can increase the amount of sex hormones, sperm production, and fertility, and also prevent the testicles from shrinking and increase the amount of semen (8).
Among the aerobic exercises, low-intensity swimming aerobic exercise is one of the exercises that is safe and usable in various physiological conditions and is used in most physiological, biochemical, and molecular reaction studies due to its intolerance to aquatic compared to non-aquatic sports. On the other hand, no study has been found to investigate the simultaneous effects of swimming, cell therapy, and laser therapy on azoospermic rats. Therefore, this study aims to investigate whether regular aerobic exercise, laser therapy, and cell therapy affect the expression of genes involved in mitochondrial dynamics of testicular tissue in azoospermic rats.
Methods
In the present experimental research, 40 male Wistar rats (6-8-week-old) were purchased from the Center for Research and Reproduction of Laboratory Animals in Tehran after transferring the subjects to the laboratory and after one week. Adaptation to the new environment was maintained in groups of 5 in transparent polycarbonate cages in an environment with a mean temperature of 22 ± 1.4 °C, humidity of 55%, and a dark cycle of 12:12 hours. The care of the animals was carried out in accordance with the guidelines of the International Institute of Health and the protocols of this study, in accordance with the principles of the Helsinki Declaration, and the rules of medical ethics. Also, during the research, the animals were fed with a food pack (Made by Behparvar Karaj Company) at the rate of 10 g per 100 g body weight per day (According to weekly weight gain) and had free access to drinking water through bottles.
In order to create an azoospermia model, busulfan at a dose of 40 mg /kg body weight was then injected intraperitoneally into each rat (the waiting time for creating the model was 30 days) (9).
One month after model induction, rats were grouped as follows:1) Healthy control group (Maintained for 8 weeks); 2) sham group; 3) patient group (Remained for 8 weeks until the end of the study; 4) patient + laser group (One month after the model was developed, a low-power laser with a wavelength of 632.8 nm, a power of 10 mW, and an energy of 3 joules was applied to the testes of azoospermic rats in three repetitions throughout the study period at weekly intervals, and rats were kept for 8 weeks until the end of the study; 5) patient + exercise group (One month after azoospermia, the rats swam with low intensity for 30 minutes a day, 5 days a week, for 8 weeks; 6) patient + cell group (One month after azoospermia, once stem cells were transplanted in the vas deferens at the rate of one million cells per rat in the right testicle, and the rats were kept for 8 weeks until the end of the study); 7) patient + exercise + laser group (One month after azoospermia, low-power laser with a wavelength of 632.8 nm, a power of 10 mW, and an energy of 3 joules was applied in three repetitions throughout the study period with an interval of once a week. Then, after one week, rats swam for 30 minutes a day, for 5 days a week, for 8 weeks); and 8) patient + cell + exercise group (One month after azoospermia, once stem cells were transplanted in the vas deferens at the rate of one million cells per rat. Then, after one week of transplantation cells, rats swam for 30 minutes a day, for 5 days a week, for 8 weeks).
Before starting the main protocol, the rats in the exercise groups were placed in a water pool for 20 minutes, each time for one week (5 days), in order to being familiarized with the water, reduce the stress of swimming, and adapt to the exercise conditions. Then, they swam 5 days a week until the end of the research period in a water tank with dimensions of 50 x 50 x 100 cm with a temperature of 30-32℃ for 8 weeks. The duration of exercise in water was 30 minutes daily until the end of the exercise period (1).
Sampling of rats’ testicular tissues was performed under very similar conditions and in baseline conditions (Two days after the end of the exercise period). In order to eliminate the acute effects of exercise, the animals were sampled 48 hours following the last swimming exercise program. For this purpose, the animals were first anesthetized using the peritoneal injection of ketamine (50-30 mg / kg) and xylazine (3-5 mg / kg) and then killed. After killing the transplanted tissues, they were evaluated for genetic studies.
To investigate the expression of the studied genes in each group, tissue analysis was performed using the real-time polymerase chain reaction (RT-PCR) technique. First, primer design was performed, and then total RNA was extracted from tissues and converted to cDNA. The cDNA was then amplified by PCR and examined for the expression of the mentioned genes. For molecular studies on the gene expression level, RNA was extracted from tissues in all study groups according to the manufacturer’s protocol (Kiagen, Germany). After extracting RNA with high purity and concentration from all samples, cDNA was synthesized according to the manufacturer’s protocol (Fermentas, USA), and then the synthesized cDNA was used for a reverse transcription reaction. For the real-time quantitative PCR (RT-qPCR) technique, first, the RNA of all cells was extracted according to the synagen protocol using chiazol solution and exposed to deoxyribonuclease I (DNase I) (Fermentas) to ensure contamination with genomic DNA. Then, the quality of the extracted RNAs was evaluated by a spectrophotometric device (DPI-1, Kiagen). To prepare a single-stranded cDNA from Oligodt primer (MWG-Biotech, Germany) and reverse transcription enzyme (Fermentas) was performed according to the relevant protocol. Each PCR reaction was performed using PCR master mix Applied Biosystems and SYBER Green in the device ABI Step One (Applied Biosystems, Sequences Detection Systems Focter City, CA.) according to the manufacturer's protocol. Forty cycles were considered for each RT-PCR cycle, and the temperatures of each cycle were set, including 94 °C for 20 seconds, 60-58 °C for 30 seconds, and 72 °C for 30 seconds. A melting diagram was performed to evaluate the accuracy of PCR reactions, and it was evaluated specifically for each gene and in each reaction, with a negative control diagram to check for contamination in each reaction.
To analyze the findings of this study, the Kolmogorov-Smirnov test, Fisher’s one-way analysis of variance (ANOVA), and Tukey’s test were used for comparison between different groups. All calculations were performed using SPSS statistical software version 22 at a significant level of P ≥ 0.05.
Results
Examining the mean and standard deviation of Mfn2 levels in different research groups shows a significant difference in Mfn2 between research groups (F=4.197; P=0.002). The highest Mfn2 index levels belonged to the laser-exercise group, and the lowest levels belonged to the patient group. The results of the one-way ANOVA on the levels of Mfn2 in different groups indicate a significant difference in the Mfn2 levels between different groups. Induction of the experimental model of azoospermia decreased the Mfn2 gene expression in the testicular tissue compared to the healthy control group. The simultaneous implementation of laser + exercise significantly increased the expression of this gene compared to the patient group (P=0.004) (Table 1; Figure 1).
Examining the mean and standard deviation of Drp1 levels in different research groups shows no significant differences in Drp1 between research groups (F=2.577; P=0.057). The highest levels of the Drp1 index belonged to the sham group, and the lowest belonged to the healthy control group. The results of the one-way ANOVA in Drp1 levels between different research groups show no significant difference in the Drp1 levels between different groups (Table 2; Figure 2).
Table 1. The results of one-way analysis of variance of mitofusin 2 (Mfn2) levels in different research groups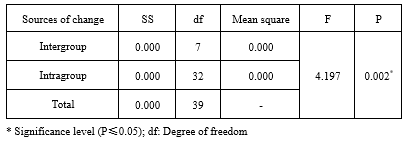 |
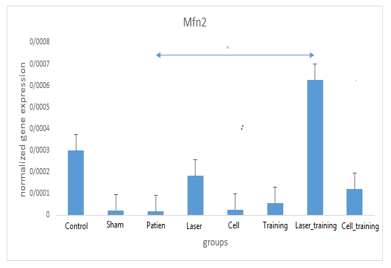 Figure 1. Comparison of mean mitofusin 2 (Mfn2) levels between different research groups * Significant signs in relation to the patient group and significant signs compared to the healthy group Table 2. The results of one-way analysis of variance of dynamin-related protein 1 (Drp1) levels in different research groups .PNG) .PNG) Figure 2. Comparison of mean dynamin-related protein 1 (Drp1) levels between different research groups |
Examining the mean and standard deviation of Mfn2 levels in different research groups shows a significant difference in Mfn2 between research groups (F=4.303; P=0.002). The highest Murf1 index levels belonged to the patient group, and the lowest belonged to the healthy control group. The results of the one-way ANOVA in the Murf1 levels in different research groups indicate a significant difference in the Murf1 levels in different research groups (Table 3). The induction of the experimental model of azoospermia significantly increased Murf1 gene expression compared to the healthy control group (p=0.003). The implementation of each of the interventions, including cell (p=0.046), exercise (p=0.003), laser + exercise (p=0.003), and cell + exercise, significantly decreased the expression of this gene compared to the patient group (p=0.004) (Table 3; Figure 3).
|
Table 3. The results of one-way analysis of variance of muscle-specific RING finger protein 1 (Murf1) levels in different research groups
.PNG) |
.PNG) Figure 3. Comparison of mean muscle-specific RING finger protein 1 (Murf1) levels between different research groups * Significant signs in relation to the patient group and significant signs compared to the healthy group |
Discussion
The induction of the azoospermia model caused a significant decrease in testicular tissue Mfn2 gene, which was enhanced by exercise, laser, cell, and combination methods in testicular tissue, which was only significant in the exercise + laser group. Regarding Drp1 gene expression in rats’ testicular tissues, the induction of the azoospermia model increased the expression of this gene, but the treatment methods used decreased the expression of this gene, which was not significant in any of the groups. Also, the induction of the azoospermia model significantly increased the Murf1 gene expression in the testicular tissue, but the treatment methods decreased the expression of this gene, which was significant in the exercise, cell, exercise + laser, exercise + cell, and cell + laser groups.
Muzaffar et al. showed a significant improvement in spermatogenesis and testicular tissue indices in samples treated with culture medium obtained from bone marrow mesenchymal stem cells, compared to the induced azoospermic group (6). Other studies in animals, such as hamsters and mice, have demonstrated a significant improvement in spermatogenesis due to stem cell therapy (10,11). On the other hand, studies have shown that many cellular pathways are regulated by cell redox status. Modulation of cellular redox status by nuclear factor kappa B (NF-κB) transcription factor and phospholipase A2 increases DNA synthesis. Therefore, low-power laser accelerates the rhythm of mitotic and meiotic divisions in spermatogenesis and increases the number of germ cells, especially primary spermatocytes (7). In addition to affecting oxidative phosphorylation in mitochondria and increasing ATP production, low-power laser beams increase microcirculation (Microscopic circulation). Laser causes vasodilation by releasing chemicals, such as histamine, which enhances cellular metabolism along with increasing oxidative phosphorylation of cells (1, 7). Increased microcirculation in the testes improves the metabolic status, which, after laser irradiation, justifies the important biological stimulatory effect on spermatogenesis (12).
Also, based on the results of previous research, exercise has positive effects on mitochondrial health (13). Zeng et al. showed a significant increase in Mfn2 protein related to skeletal muscle mitochondrial fusion in elderly mice after exercise interventions (14). Also, comparing the mitochondrial fusion stress response to exercise activity, studies have shown that the expression of genes associated with mitochondrial cleavage significantly increases after prolonged exercise (15). According to Fealy et al.’s study, exercise reduces the activity of Drp1 protein in skeletal muscle (16). One of the properties of Drp1 is apoptosis in mitochondria, so exercise can protect tissues against oxidative stress and reduce Drp1 levels by reducing fat peroxides and activating antioxidant defenses. Decreased Drp1 reduces cleavage and apoptosis in cells and improves apoptotic-dependent markers (17). The reduction of Drp1 is essential for the development and maintenance of proper mitochondrial function, and it improves cell survival by reducing oxidative stress (18).
The ubiquitin-proteasome pathway is applied to the entire cell and involves many substrates and reaction processes in vivo, including the ubiquitination of substrate proteins and the degradation of ubiquitinated proteins. Ubiquitin E3 ligases, such as atrogin-1 and Murf1, are essential for protein ubiquitination (19). In Zheng’s research, exercise significantly reduced Murf1 gene expression in the skeletal muscle of older rats, which may subsequently inhibit the process of protein ubiquitination (14). The regulation of Murf1, including the NF-κB and forkhead box O3a (FoxO3a) signaling pathways, has a cumulative effect on skeletal muscle atrophy, with NF-κB being one of the most important transcription factors for the regulation of Murf1 involved in senile skeletal muscle atrophy (20). Exercise can reduce the expression of Murf1 proteins mediated by inhibiting oxidative stress (21,22).
In this study, each of the treatment method was effective in mitochondrial dynamics, but in each of the combined methods, a better result was obtained, and they had a synergistic effect, and this synergy was more in the laser + exercise group than in other combined methods.
Conclusion
According to the research results, it is possible that the combination of swimming exercise with cell therapy and laser therapy by changing some of the genes involved in the mitochondrial dynamics of the testicular tissue of experimental rats with azoospermia may cause the rats to become fertile, but a definite opinion needs more research in this regard.
Acknowledgement
This research was extracted from a doctoral dissertation at Islamic Azad University, Aliabad Katoul Branch. The authors hereby express their gratitude and appreciation to this academic center.
Funding sources
This article received no funding and was conducted at the personal expense of the authors.
Ethical statement
All steps of the present research have been approved by the Research Ethics Committee of Islamic Azad University, Sari Branch (IR.IAU.SARI.REC.1398.149).
Conflicts of interest
No conflict of interest.
Author contributions
All authors contributed equally to the writing of this article.
Research Article: Research Article |
Subject:
Sport Physiology
Received: 2022/12/26 | Accepted: 2023/11/6 | Published: 2024/08/3 | ePublished: 2024/08/3
Received: 2022/12/26 | Accepted: 2023/11/6 | Published: 2024/08/3 | ePublished: 2024/08/3
References
1. Shapouri A, Asgharpour H, Farzanegi P, Aghaei Bahmanbeglo N. Regulation of increased expression of genes involved in mitochondrial dynamics of testicular tissue (PGC1-α and OPA1) in azoospermia model rats in interventions based on laser and physical activity. Jundishapur Scientific Medical Journal. 2024;23(1):89-102. [View at Publisher] [DOI] [Google Scholar]
2. Sun J, Brown TT, Samuels DC, Hulgan T, D'Souza G, Jamieson BD, et al. The role of mitochondrial DNA variation in age-related decline in gait speed among older men living with human immunodeficiency virus. Clinical Infectious Diseases. 2018; 67(5): 778-84. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Tang W-X, Wu W-H, Qiu H-Y, Bo H, Huang S-M. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. Journal of nephrology. 2013; 26(6): 1073-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. San-Millán I. The key role of mitochondrial function in health and disease. Antioxidants. 2023;12(4):782. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, et al. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. International journal of molecular sciences. 2014; 15(8): 13151-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Mozafar A, Mehranani D, Vahdati A, Hosseini S, Forouzanfar M. The Effect of Bone Marrow Mesenchymal Stem Cell Culture in the Treatment of Azoospermic Infertility induced by Busulfan Balb/C mice. Armaghane Danesh. 2017; 22(3): 295-310. [View at Publisher] [Google Scholar]
7. Deihimi M, Azornia M, Takzare N. Effect of red and infrared spectrum low level of laser rays on Rat Seminiferous tubules. Journal of Gorgan University of Medical Sciences. 2010; 12(3): 10-7. [View at Publisher] [Google Scholar]
8. Zohrabi Karani L, Farzanegi P, Azarbayjani MA. The Effect of 8-Weeks of Low-Intensity Swimming Training on Promyelocytic Leukemia Zinc Finger Protein and Spermatid Transition Nuclear Protein Gene Expression in Azoospermic Rats Model. Internal Medicine Today. 2020; 26(4): 332-47. [View at Publisher] [DOI] [Google Scholar]
9. Abougalala FMA, Ali EK, Fayyad RMA, Elsaied MY, Abdelmonsef AS. Mesenchymal stem cells for Busulfan-Induced Azoospermia: An experimental study. International Journal of Medical Arts. 2022;4(4):2319-24. [View at Publisher] [DOI] [Google Scholar]
10. Abdelaal NE, Tanga BM, Abdelgawad M, Allam S, Fathi M, Saadeldin IM, et al. Cellular therapy via spermatogonial stem cells for treating impaired spermatogenesis, non-obstructive azoospermia. Cells. 2021;10(7):1779. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, et al. Recovery of fertility in azoospermia rats after injection of adipose‐tissue‐derived mesenchymal stem cells: the sperm generation. BioMed research international. 2013;2013(1):529589. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, et al. Phenotypic plasticity of mouse spermatogonial stem cells. PloS one. 2009; 4(11): e7909. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. The Journal of physiology. 2021; 599(3): 803-17. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Zeng Z, Liang J, Wu L, Zhang H, Lv J, Chen N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Frontiers in physiology. 2020;11:583478. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Bori Z, Zhao Z, Koltai E, Fatouros IG, Jamurtas AZ, Douroudos II, et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Experimental gerontology. 2012; 47(6): 417-24. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. Journal of Applied Physiology. 2014; 117(3): 239-45. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Delroz H, Abdi A, Barari A, Farzanegi P. Protective Effect of aerobic training along with resveratrol on mitochondrial dynamics of cardiac myocytes in animal model of non-alcoholic fatty liver disease. Journal of Ardabil University of Medical Sciences. 2019; 19(3): 272-83. [View at Publisher] [DOI] [Google Scholar]
18. Fukumitsu K, Hatsukano T, Yoshimura A, Heuser J, Fujishima K, Kengaku M. Mitochondrial fission protein Drp1 regulates mitochondrial transport and dendritic arborization in cerebellar Purkinje cells. Molecular and Cellular Neuroscience. 2016; 71: 56-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Pang Z. Functions of the ubiquitin system in mammalian spermatogenesis and skeletal muscle. 2011. [View at Publisher] [Google Scholar]
20. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. American Journal of Physiology-Endocrinology and Metabolism. 2014; 307(6): E469-E84. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Cunha TF, Bacurau AV, Moreira JB, Paixão NA, Campos JC, et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS One. 2012; 7(8): e41701. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Chen G-Q, Mou C-Y, Yang Y-Q, Wang S, Zhao Z-W. Exercise training has beneficial anti-atrophy effects by inhibiting oxidative stress-induced MuRF1 upregulation in rats with diabetes. Life sciences. 2011; 89(1-2): 44-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








