Volume 17, Issue 5 (Sep-Oct 2023)
mljgoums 2023, 17(5): 23-25 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
vaez A, Razavi Niko H, Hosseini S D, Mobasheri E, Tabarraei A. Hepatitis B virus in cervicovaginal lavage samples of pregnant women in Gorgan city, north of Iran. mljgoums 2023; 17 (5) :23-25
URL: http://mlj.goums.ac.ir/article-1-1463-en.html
URL: http://mlj.goums.ac.ir/article-1-1463-en.html
1- Department of Microbiology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
2- Congenital Malformations Research Center,Golestan University of Medical Sciences, Gorgan, Iran
3- Infectious Diseases Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,tabarraei@goums.ac.ir
2- Congenital Malformations Research Center,Golestan University of Medical Sciences, Gorgan, Iran
3- Infectious Diseases Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,
Full-Text [PDF 438 kb]
(1304 Downloads)
| Abstract (HTML) (4915 Views)
Full-Text: (943 Views)
Introduction
The hepatitis B virus (HBV) is the cause of acute or chronic liver infection that has become a significant public health problem nowadays (1). In 2016, the World Health Organization (WHO) reported that HBV had infected approximately 2 billion people. More than 257 million suffer from chronic liver disease, and 887 000 deaths occur annually due to acute and chronic liver dysfunction worldwide (2, 3). It is estimated that the number of people infected with HBV in Iran is approximately 1.5 million (4). The virus is spread through vertical transmission from mother to fetus, during pregnancy, and mostly at birth. Also, transfusion of infected blood and blood compounds, sexual intercourse, and close and long-term contact with infected carriers are the ways of horizontal transmission of this virus (5). In intermediate-prevalence (3%-5%) regions, including the Middle East and countries like Iran, sexual transmission and vertical transmission are the major routes of infection (6).
The World Health Organization has recommended screening for HBV infection in pregnant women due to the goal of eliminating viral hepatitis as a major public health threat by 2030 (7, 8). However, this goal cannot be achieved without solving the problem of vertical transmission of HBV. The infection risk among infants born to women with hepatitis B surface antigen (HBsAg) and HBeAg positive and high levels of serum viral DNA (≥8 log copies/mL) is up to 90%, and over 85% of them become chronic carriers (9, 10). Furthermore, approximately 25% of these children die primarily because of cirrhosis and hepatocellular carcinoma (9). Hepatitis B virus diagnosis during pregnancy or before delivery is the only possible way to prevent vertical transmission. Therefore, effective and rapid diagnosis is key to controlling the infection and establishing prevention programs for disease (11).
The regular specimen chosen for the diagnosis of HBV infection in pregnant women is venous blood. However, due to the aims of studies or medical purposes, it could be placenta blood, vaginal secretion, amniotic fluid, and, in some cases, oocyte, plasma of ovum, and interstitial cells (12).
Serological tests for viral antigens and antibodies are typically used for diagnostic screening on either serum or plasma; however, molecular diagnosis is highly sensitive to serological tests, making the virus genome detectable about 3-5 weeks after infection (on average 6-15 days before the onset of HBsAg). The genome may also be identifiable in the blood years after acute hepatitis has improved (13-15). This study is due to the lack of similar study on vaginal discharge in this city; in addition, according to the results mentioned above about the time of infection and the possibility of a negative serum response in the early stages of infection, as well as the possibility of infection transmission through the vagina or oocytes, this study aimed to assess the presence of HBV in pregnant women's vaginal secretion referred to Sayyad Shirazi Hospital in Gorgan City, north of Iran.
Methods
In this cross-sectional study, 315 cervicovaginal lavage samples were collected from pregnant women referred to Sayyad Shirazi Hospital in Gorgan City, north of Iran. The study was approved by the Research Ethics Committee of Golestan University of Medical Sciences, Iran (code: IR.GOUMS.REC.1397.070). Liquid samples were transported on ice to the Microbiology Department's laboratory at Golestan University of Medical Sciences, Gorgan, Iran. Viral DNA was extracted from samples using the Bioneer ExiPrep Plus Viral DNA/RNA Kit. The quality of each extracted DNA was checked by agarose gel electrophoresis, and each concentration was measured using a NanoDrop spectrophotometer (Thermo Scientific); then, extraction accuracy was confirmed using GAPDH polymerase chain reaction (PCR). Participants’ clinical, demographic, and behavioral data were collected.
The polymerase chain reaction was performed in a total volume of 20 μL using a Super PCR Master Mix (Cat No. YT1553, Yekta Tajhiz Azma, Iran) and primers designed by Primer 3 Input version 0.4.0, which are as follows: forward: 5 '-CGGAACATTGTTCACCTC-3' and
reverse: 5'-GGCCCATATTAACATTGACATA-3'. Polymerase chain reaction cycling was performed on an Eppendorf Master Cycler (Germany). Cycling condition was set up using positive control collected from the Kavosh Medical Diagnosis Laboratory, Gorgan, and performed as follows: 95 °C for 5 minutes as initial denaturation, 94 °C for 1 minute, 54.6 °C for 1 minute, and 72 °C for 1 minute for 35 cycles, and 72 °C for 5 minutes as a final extension. The presence of HBV DNA was determined after electrophoresis on 1.5% agarose gels containing Tris-borate-EDTA (TBE) buffer and visualized directly under ultraviolet illumination using a gel documentation system. A 50-base pairs (bp) ladder was used to score the band sizes.
Demographic, clinical, behavioral, and molecular data were entered into SPSS version 16 (SPSS Inc, Chicago, IL, USA). The chi-square tests were used to determine any association between categorical data. Risk estimation analysis was performed for the univariate analysis.
Results
A total of 315 pregnant women were examined in the maternity ward of Shahid Sayyad Shirazi Educational and Medical Center in Gorgan City from June to October 2018. The age range of patients was 14 to 43 years, with an average of
The population consisted of 94% (296/315) of pregnant housewives and 6% (19/315) of employees. In addition, 54.6% (172/315) of the participants were from urban areas and 45.1% (142/315) from rural areas.
Hepatitis B virus DNA was detected in 2.2% (7/315) of cases. Further, 57.13% (4/7) were less than 30 years old, and 42.87% (3/7) were older; in addition, 1.26% (4/315) of HBV DNA-positive cases were from urban areas and 0.95% (3/315) from rural areas. None of the cases were illiterate. All of the infected pregnant women were in the third trimester of pregnancy. Only 1 case had a history of miscarriage. In addition, 115 (38.7%) had a history of unusual discharge, 4 of whom had HBV DNA. Only one of the infected women (14.28%) had a history of blood transfusion.
No statistical correlation was seen between clinical and demographic data and the presence of HBV DNA in pregnant women of Gorgan.
Among HBV-positive pregnant women, 4 cases never used condoms as infection protection, and 3 cases had anal sex. These behavioral risk factors are significantly associated with the HBV presence in pregnant women, respectively (P = 0.043 and P = 0.047); more data are shown in (Table 1).
Discussion
In this study, the presence of HBV DNA among pregnant women’s cervicovaginal samples referred to Sayyad Shirazi Hospital in Gorgan was 2.2%. Not using a condom (P = 0.043) and anal sex (P = 0.047) were significantly associated with the presence of HBV in these pregnant women. No significant correlation was observed between demographical and clinical data in HBV-positive cases.
The seroepidemiological prevalence of HBV is very different worldwide. According to a 2015 report, the African region had the highest prevalence of HBV infection (8.83%), while the Americas region had the lowest prevalence (0.81%) (1). In the last 2 decades, HBV infection among Iranian pregnant women has shown varying prevalence rates. For instance, in Zahedan City in 2005, the prevalence was reported as 6.5% (16), while it was 0.16% in Babol City in 2011 (17). More recently, in Sari City in 2021, the prevalence was reported as 1.57% (4). Also, the prevalence varied in Gorgan City from 1.2% in 2000 (18) to 1% in 2011 (19). The latest systematic review (2016) reported an HBV prevalence of 2.2% in the general Iranian population (20). Our molecular genomic test results showed 2.2% of the HBV DNA presence in the vaginal secretions of Gorgan’s pregnant women, which is consistent with the prevalence of HBV in the general population of Iran. However, the varied prevalence of HBV in different provinces can be related to the study’s aims, molecular or serological diagnosis, type of sampling, endemicity of HBV in some areas, and the lack of good health and treatment services in some underdeveloped areas. As there is not enough data on the presence of HBV in the vaginal secretions of pregnant women and the importance of understanding the transmission of this virus to neonates and women’s partners, our results could be useful for implementing preventive measures in this subject.
There were 3 and 4 infected women who never used condoms as infection protection and with a history of anal sex, respectively. A significant correlation was observed between these 2 behavioral factors and the presence of HBV DNA in pregnant women. A recent study conducted on pregnant women in Babol City, north of Iran, showed a significant relationship between using condoms and a history of HBV infection in the family (4).
In contrast to some studies reported HBV-positive women with lower rates of virus in urban areas and less than 30 years age, our result showed a higher infection rate in under 30-year-old women that were residents in rural areas. (4, 21). The relatively low prevalence of HBV among pregnant women under 30 years old in the 2 studies mentioned above may be attributed to the initiation of the HBV vaccination program in Iran in 1993. Vaginal secretions sample instead of blood in this study with higher rate of HBV DNA positive cannot be a definitive reason for the existence of the disease in under 30-year-old women. Overall, the lack of proper vaccination in rural and distant areas of the city, lack of equal good health care services throughout Iran, and lack of vaccination in some areas of the region in the early years could be some debatable hypothetical reasons for defining this difference.
As mentioned in (Table 1), only 1 woman with HBV DNA (1/7) showed a history of blood transfusion; there is no statistical correlation between this risk factor and the presence of HBV DNA. In contrast, a systematic review and meta‑analysis study (1900-2016) in Iranian pregnant women (11) and a study from south Khorasan Province (22) reported a significant relationship.
However, in the meta‑analysis study, several risk factors were found to be significantly associated with the higher prevalence of HBV infection in Iranian pregnant women, including illiteracy, abortion, blood transfusion, and addicted spouses. Also, some factors such as urbanization, occupation, history of surgery, or tattooing showed no statistical relationship. None of the risk factors mentioned earlier are significantly associated with HBV DNA-positive in pregnant women in our study (11).
Conclusion
Hepatitis B virus DNA was found in the vaginal secretions of Gorgan’s pregnant women. The prevalence of HBV infection among individuals with a 2.2% positivity rate may be considered similar to the general prevalence of HBV infection. However, it holds significant importance within this specific group of people due to the potential for vertical and horizontal transmission of the virus to neonates and their partners. It is strongly recommended to conduct screening and provide management for women during pregnancy.
Acknowledgement
This research was financially supported by the Infectious Diseases Research Center and Research Assistant of Golestan University of Medical Sciences.
Funding sources
No funding was used for this study.
Ethical statement
An informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was approved by the Research Ethics Committee of Golestan University of Medical Sciences, Iran (code: IR.GOUMS.REC.1397.070).
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
All author of this article has cooperated in study design, collecting samples, conducting tests, and writing and editing of the manuscript.
The hepatitis B virus (HBV) is the cause of acute or chronic liver infection that has become a significant public health problem nowadays (1). In 2016, the World Health Organization (WHO) reported that HBV had infected approximately 2 billion people. More than 257 million suffer from chronic liver disease, and 887 000 deaths occur annually due to acute and chronic liver dysfunction worldwide (2, 3). It is estimated that the number of people infected with HBV in Iran is approximately 1.5 million (4). The virus is spread through vertical transmission from mother to fetus, during pregnancy, and mostly at birth. Also, transfusion of infected blood and blood compounds, sexual intercourse, and close and long-term contact with infected carriers are the ways of horizontal transmission of this virus (5). In intermediate-prevalence (3%-5%) regions, including the Middle East and countries like Iran, sexual transmission and vertical transmission are the major routes of infection (6).
The World Health Organization has recommended screening for HBV infection in pregnant women due to the goal of eliminating viral hepatitis as a major public health threat by 2030 (7, 8). However, this goal cannot be achieved without solving the problem of vertical transmission of HBV. The infection risk among infants born to women with hepatitis B surface antigen (HBsAg) and HBeAg positive and high levels of serum viral DNA (≥8 log copies/mL) is up to 90%, and over 85% of them become chronic carriers (9, 10). Furthermore, approximately 25% of these children die primarily because of cirrhosis and hepatocellular carcinoma (9). Hepatitis B virus diagnosis during pregnancy or before delivery is the only possible way to prevent vertical transmission. Therefore, effective and rapid diagnosis is key to controlling the infection and establishing prevention programs for disease (11).
The regular specimen chosen for the diagnosis of HBV infection in pregnant women is venous blood. However, due to the aims of studies or medical purposes, it could be placenta blood, vaginal secretion, amniotic fluid, and, in some cases, oocyte, plasma of ovum, and interstitial cells (12).
Serological tests for viral antigens and antibodies are typically used for diagnostic screening on either serum or plasma; however, molecular diagnosis is highly sensitive to serological tests, making the virus genome detectable about 3-5 weeks after infection (on average 6-15 days before the onset of HBsAg). The genome may also be identifiable in the blood years after acute hepatitis has improved (13-15). This study is due to the lack of similar study on vaginal discharge in this city; in addition, according to the results mentioned above about the time of infection and the possibility of a negative serum response in the early stages of infection, as well as the possibility of infection transmission through the vagina or oocytes, this study aimed to assess the presence of HBV in pregnant women's vaginal secretion referred to Sayyad Shirazi Hospital in Gorgan City, north of Iran.
Methods
In this cross-sectional study, 315 cervicovaginal lavage samples were collected from pregnant women referred to Sayyad Shirazi Hospital in Gorgan City, north of Iran. The study was approved by the Research Ethics Committee of Golestan University of Medical Sciences, Iran (code: IR.GOUMS.REC.1397.070). Liquid samples were transported on ice to the Microbiology Department's laboratory at Golestan University of Medical Sciences, Gorgan, Iran. Viral DNA was extracted from samples using the Bioneer ExiPrep Plus Viral DNA/RNA Kit. The quality of each extracted DNA was checked by agarose gel electrophoresis, and each concentration was measured using a NanoDrop spectrophotometer (Thermo Scientific); then, extraction accuracy was confirmed using GAPDH polymerase chain reaction (PCR). Participants’ clinical, demographic, and behavioral data were collected.
The polymerase chain reaction was performed in a total volume of 20 μL using a Super PCR Master Mix (Cat No. YT1553, Yekta Tajhiz Azma, Iran) and primers designed by Primer 3 Input version 0.4.0, which are as follows: forward: 5 '-CGGAACATTGTTCACCTC-3' and
reverse: 5'-GGCCCATATTAACATTGACATA-3'. Polymerase chain reaction cycling was performed on an Eppendorf Master Cycler (Germany). Cycling condition was set up using positive control collected from the Kavosh Medical Diagnosis Laboratory, Gorgan, and performed as follows: 95 °C for 5 minutes as initial denaturation, 94 °C for 1 minute, 54.6 °C for 1 minute, and 72 °C for 1 minute for 35 cycles, and 72 °C for 5 minutes as a final extension. The presence of HBV DNA was determined after electrophoresis on 1.5% agarose gels containing Tris-borate-EDTA (TBE) buffer and visualized directly under ultraviolet illumination using a gel documentation system. A 50-base pairs (bp) ladder was used to score the band sizes.
Demographic, clinical, behavioral, and molecular data were entered into SPSS version 16 (SPSS Inc, Chicago, IL, USA). The chi-square tests were used to determine any association between categorical data. Risk estimation analysis was performed for the univariate analysis.
Results
A total of 315 pregnant women were examined in the maternity ward of Shahid Sayyad Shirazi Educational and Medical Center in Gorgan City from June to October 2018. The age range of patients was 14 to 43 years, with an average of
The population consisted of 94% (296/315) of pregnant housewives and 6% (19/315) of employees. In addition, 54.6% (172/315) of the participants were from urban areas and 45.1% (142/315) from rural areas.
Hepatitis B virus DNA was detected in 2.2% (7/315) of cases. Further, 57.13% (4/7) were less than 30 years old, and 42.87% (3/7) were older; in addition, 1.26% (4/315) of HBV DNA-positive cases were from urban areas and 0.95% (3/315) from rural areas. None of the cases were illiterate. All of the infected pregnant women were in the third trimester of pregnancy. Only 1 case had a history of miscarriage. In addition, 115 (38.7%) had a history of unusual discharge, 4 of whom had HBV DNA. Only one of the infected women (14.28%) had a history of blood transfusion.
No statistical correlation was seen between clinical and demographic data and the presence of HBV DNA in pregnant women of Gorgan.
Among HBV-positive pregnant women, 4 cases never used condoms as infection protection, and 3 cases had anal sex. These behavioral risk factors are significantly associated with the HBV presence in pregnant women, respectively (P = 0.043 and P = 0.047); more data are shown in (Table 1).
|
Table 1. The clinical, demographic, and behavioral data of Gorgan’s pregnant women
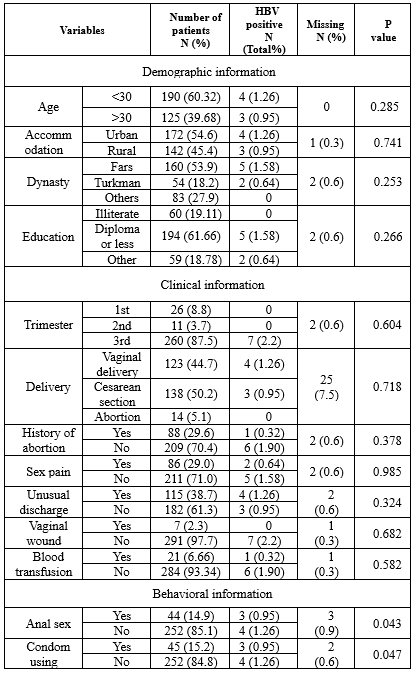 |
Discussion
In this study, the presence of HBV DNA among pregnant women’s cervicovaginal samples referred to Sayyad Shirazi Hospital in Gorgan was 2.2%. Not using a condom (P = 0.043) and anal sex (P = 0.047) were significantly associated with the presence of HBV in these pregnant women. No significant correlation was observed between demographical and clinical data in HBV-positive cases.
The seroepidemiological prevalence of HBV is very different worldwide. According to a 2015 report, the African region had the highest prevalence of HBV infection (8.83%), while the Americas region had the lowest prevalence (0.81%) (1). In the last 2 decades, HBV infection among Iranian pregnant women has shown varying prevalence rates. For instance, in Zahedan City in 2005, the prevalence was reported as 6.5% (16), while it was 0.16% in Babol City in 2011 (17). More recently, in Sari City in 2021, the prevalence was reported as 1.57% (4). Also, the prevalence varied in Gorgan City from 1.2% in 2000 (18) to 1% in 2011 (19). The latest systematic review (2016) reported an HBV prevalence of 2.2% in the general Iranian population (20). Our molecular genomic test results showed 2.2% of the HBV DNA presence in the vaginal secretions of Gorgan’s pregnant women, which is consistent with the prevalence of HBV in the general population of Iran. However, the varied prevalence of HBV in different provinces can be related to the study’s aims, molecular or serological diagnosis, type of sampling, endemicity of HBV in some areas, and the lack of good health and treatment services in some underdeveloped areas. As there is not enough data on the presence of HBV in the vaginal secretions of pregnant women and the importance of understanding the transmission of this virus to neonates and women’s partners, our results could be useful for implementing preventive measures in this subject.
There were 3 and 4 infected women who never used condoms as infection protection and with a history of anal sex, respectively. A significant correlation was observed between these 2 behavioral factors and the presence of HBV DNA in pregnant women. A recent study conducted on pregnant women in Babol City, north of Iran, showed a significant relationship between using condoms and a history of HBV infection in the family (4).
In contrast to some studies reported HBV-positive women with lower rates of virus in urban areas and less than 30 years age, our result showed a higher infection rate in under 30-year-old women that were residents in rural areas. (4, 21). The relatively low prevalence of HBV among pregnant women under 30 years old in the 2 studies mentioned above may be attributed to the initiation of the HBV vaccination program in Iran in 1993. Vaginal secretions sample instead of blood in this study with higher rate of HBV DNA positive cannot be a definitive reason for the existence of the disease in under 30-year-old women. Overall, the lack of proper vaccination in rural and distant areas of the city, lack of equal good health care services throughout Iran, and lack of vaccination in some areas of the region in the early years could be some debatable hypothetical reasons for defining this difference.
As mentioned in (Table 1), only 1 woman with HBV DNA (1/7) showed a history of blood transfusion; there is no statistical correlation between this risk factor and the presence of HBV DNA. In contrast, a systematic review and meta‑analysis study (1900-2016) in Iranian pregnant women (11) and a study from south Khorasan Province (22) reported a significant relationship.
However, in the meta‑analysis study, several risk factors were found to be significantly associated with the higher prevalence of HBV infection in Iranian pregnant women, including illiteracy, abortion, blood transfusion, and addicted spouses. Also, some factors such as urbanization, occupation, history of surgery, or tattooing showed no statistical relationship. None of the risk factors mentioned earlier are significantly associated with HBV DNA-positive in pregnant women in our study (11).
Conclusion
Hepatitis B virus DNA was found in the vaginal secretions of Gorgan’s pregnant women. The prevalence of HBV infection among individuals with a 2.2% positivity rate may be considered similar to the general prevalence of HBV infection. However, it holds significant importance within this specific group of people due to the potential for vertical and horizontal transmission of the virus to neonates and their partners. It is strongly recommended to conduct screening and provide management for women during pregnancy.
Acknowledgement
This research was financially supported by the Infectious Diseases Research Center and Research Assistant of Golestan University of Medical Sciences.
Funding sources
No funding was used for this study.
Ethical statement
An informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was approved by the Research Ethics Committee of Golestan University of Medical Sciences, Iran (code: IR.GOUMS.REC.1397.070).
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
All author of this article has cooperated in study design, collecting samples, conducting tests, and writing and editing of the manuscript.
Research Article: Research Article |
Subject:
Virology
Received: 2021/11/21 | Accepted: 2021/11/24 | Published: 2024/01/15 | ePublished: 2024/01/15
Received: 2021/11/21 | Accepted: 2021/11/24 | Published: 2024/01/15 | ePublished: 2024/01/15
References
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-55. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. World Health Organization.Global hepatitis report, 2017. WHO;2017. [View at Publisher] [Google Scholar]
3. Mohebbi A, Shakeri-Moghaddam A, Doudazndegan Y, Lorestani N, Mir-Arab A, Moradi A, et al. Hepatitis B virus x protein coding sequence variation in chronically infected patient. J Gorgan Univ Med Sci. 2017;19(3):105-10. [View at Publisher] [Google Scholar]
4. Navaifar MR, Rahimzadeh G, Fahimzad AR, Safar MJ, Shamshiri AR, Rezai S, et al. Seroepidemiology of Hepatitis B in Pregnant Women in Sari, Iran 2018-2020. J Mazandaran Univ Med Sci. 2021;30(194):121-6. [View at Publisher] [Google Scholar]
5. Kasper D, Fauci A, editors. Harrison's Infectious Diseases. NewYork: McGraw-Hill Medical; 2013. [View at Publisher] [Google Scholar]
6. Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection-an update. Microb Cell. 2016;3(9):420-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Kolawole OM, Wahab AA, Adekanle DA, Sibanda T, Okoh AI. Seroprevalence of hepatitis B surface antigenemia and its effects on hematological parameters in pregnant women in Osogbo, Nigeria. Virol J. 2012;9(1):317. [View at Publisher] [Google Scholar] [DOI] [PMID] [View at Publisher] [DOI] [PMID] [Google Scholar]
8. World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. WHO; 2016. [View at Publisher] [Google Scholar]
9. Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29(S1):133-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170(6):1418-23. [View at Publisher] [Google Scholar] [DOI] [PMID] [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Badfar G, Shohani M, Nasirkandy MP, Mansouri A, Abangah G, Rahmati S, et al. Epidemiology of hepatitis B in pregnant Iranian women: a systematic review and meta-analysis. Arch virol. 2018;163(2):319-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Zhang S-L, Yue Y-F, Bai G-Q, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol. 2004;10(3):437-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Han C, Yu T, Qin W, Liao X, Huang J, Liu Z, et al. Genome-wide association study of the TP53 R249S mutation in hepatocellular carcinoma with aflatoxin B1 exposure and infection with hepatitis B virus. J Gastrointest Oncol. 2020;11(6):1333. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Villa E, Grottola A, Buttafoco P, Trande P, Merighi A, Fratti N, et al. Evidence for hepatitis B virus infection in patients with chronic hepatitis C with and without serological markers of hepatitis B. Dig Dis Sci. 1995;40(1):8-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Elkadeem M, Elnaggar R. Hepatitis B in Pregnant Females. A Cross Sectional Study in Nile Delta, Egypt. Version 1; 2021. [View at Publisher] [DOI] [Google Scholar]
16. Sharifimoud B, Kaikha F, Sanei ME, Salehi M, Alavi NR, Metanat M, et al. Epidemiological study of Hepatitis B surface antigen in pregnant women in Zahedan. zahedan journal of research in medical sciences. 2005;7(2):119-24. [View at Publisher] [Google Scholar]
17. Bayani M, Biazar T, Hasanjani RM, Bayani F, Siadati S. The effect of hepatitis B vaccination at birth on reducing the prevalence of hepatitis B surface antigen among rural pregnant women in Babol, Iran. J Babol Univ Med Sci. 2016;18(1):7-10. [View at Publisher] [Google Scholar]
18. Ahansaz M. Evaluation of HBsAg positive in pregnant women referring to prenatal ward Dezyani hospital in Gorgan in the first half of 1999 [Dissertation Thesis]. Tehran: Iran University of Medical Sciences; 2000. [Google Scholar]
19. Cheraghali F, Yazarloo S, Behnampour N, Azarhoush R. Frequency of HBsAg in pregnant women in Gorgan, Iran. J Gorgan Univ Med Sci. 2012; 13(4): 84-90. [View at Publisher] [Google Scholar]
20. Salehi-Vaziri M, Sadeghi F, Hashiani AA, Fesharaki MG, Alavian SM. Hepatitis B virus infection in the general population of Iran: an updated systematic review and meta-analysis. Hepat Mon. 2016;16(4):e35577. [View at Publisher] [DOI] [PubMed] [Google Scholar]
21. Motazakker M, Shokat Nagadeh M, Khalili F, Shayeri B. Hepatitis B virus infection among pregnant women attending health care centers of Urmia. J guilan Univ Med Sci. 2014;22(89):45-50. [View at Publisher] [Google Scholar]
22. Javanmard D, Alavian SM, Abedi F, Namaei MH, Asghari A, Ziaee M. High prevalence of hepatitis B virus infection in the Village of Esfandiar in South Khorasan Province, Iran. Hepatitis Monthly. 2018;18(8):e65473. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com