Volume 17, Issue 5 (Sep-Oct 2023)
mljgoums 2023, 17(5): 26-29 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirdar S, Khalili M, Aghaei Bahmanbeglou N. The effect of swimming and silymarin on placental growth factor in pregnant rats exposed to cadmium. mljgoums 2023; 17 (5) :26-29
URL: http://mlj.goums.ac.ir/article-1-1431-en.html
URL: http://mlj.goums.ac.ir/article-1-1431-en.html
1- Department of Sports Physiology, Faculty of Sports Sciences, Mazandaran University, Babolsar, Iran
2- Department of Sports Physiology, Islamic Azad University, Aliabad Katoul Branch, Islamic Azad University, Ali Abad Ktoul
3- Department of Physical Education and Sports Science, Ali Abad Ktoul Branch, Islamic Azad University, Ali Abad Ktoul
2- Department of Sports Physiology, Islamic Azad University, Aliabad Katoul Branch, Islamic Azad University, Ali Abad Ktoul
3- Department of Physical Education and Sports Science, Ali Abad Ktoul Branch, Islamic Azad University, Ali Abad Ktoul
Full-Text [PDF 363 kb]
(1466 Downloads)
| Abstract (HTML) (5445 Views)
Full-Text: (1207 Views)
Introduction
Pregnancy is a stressful condition in which many physiological and metabolic functions change considerably (1). In a healthy person, free radicals and antioxidants are in balance, but when this balance is disrupted, oxidative stress (OS) occurs. This OS causes tissue damage and various diseases (2). Oxidative stress plays a role in the development of health conditions, such as preterm delivery, fetal growth restriction, pregnancy poisoning, and abortion (3). Cadmium is one of the main environmental pollutants, having many effects on the surrounding environment, and even a small amount of it is very poisonous and causes cancer (4). This element has been reported to be associated with the occurrence of OS, causing reactive oxygen species (ROS) directly and indirectly. In addition, the accumulation of cadmium-induced free radicals can damage the structure and performance of different cells (including beta cells) and may result in pancreatic cancer (5,6). When cadmium passes the placenta and enters the fetus’s body, it causes different abnormalities, including cleft palate, cleft lip, non-formation of the ear or misplaced ear, and open eye (7,8). The proper use of dietary, medicinal, and herbal supplements can be helpful in minimizing the effect of cadmium. Silybum marianum is one of the vegetables that are rich in phenolic flavonolignan compounds (taxifolin, silibinin, silidianin, and silicristin) (9). The most important uses of this plant include its antioxidant, anti-inflammatory, and anticancer properties, as well as its effect on plasma lipids and lipoproteins. However, research on the inhibitory effects of Silibium marianum in contrast to the effects of exercise and physical activity on fetal growth factor is still unknown.
Placental growth factor (PLGF) is considered one of the strong angiogenic factors in tumors (10), which has been identified in inflammatory diseases, wound healing, and cancers. It is also involved in cardiovascular diseases, monocyte invasion in chronic infectious diseases, and increased internal thickness of atherosclerosis (11). Abnormal concentrations of this factor cause abnormal placental development that is observed during preeclampsia in pregnancy (12). The presence of this factor in early life after birth is essential for the growth and survival of infants, and its prevention leads to increased mortality, stunted growth, and impaired organ development (12). Angiogenesis is a process that develops in a complex way during life and adulthood. Physical exercise has been identified as an effective factor in promoting angiogenesis (13). However, few studies have been conducted on the effects of sports activities, especially swimming, on the PLGF of pregnant women who had moderate and regular aerobic exercise compared to the inactive group (14). Since it has been suggested that cadmium has an effect on the function of various cells, including endothelial cells of blood vessels and beta cells, it is possible that it has an effect on PLGF levels as well. Therefore, it is possible to use supplements
Since cadmium has been suggested to affect the function of various cells, it has been suggested that cadmium has an effect on the function of various cells, including endothelial cells of blood vessels and beta cells, it is possible that it has an effect on PLGF levels as well. Therefore, the use of supplements such as silymarin may be an effective factor in reducing the risks and diseases caused by the effect of cadmium on PLGF.
To date, no study has been reported to investigate the effect of submaximal swimming activity on PLGF concentration during pregnancy, as well as the effect of silymarin and cadmium supplementation on PLGF levels.
Methods
Seventy-two 8-week-old pregnant Wistar rats (weighing 20 ± 200 g) were divided into 9 groups, with 8 rats in each group. The rats were kept in transparent polycarbonate cages at a temperature of 23 ± 2 °C with a humidity of 45% ± 5% and light-dark cycle of 12:12. During the research period, all animals were provided ad libitum access to standard food pellets and water. One week after transportation to the laboratory and adaptation to the new environment, the animals were transferred to a swimming pool. This was done to help the rats become familiar with the water, reduce swimming-related stress, and provide them with a training environment. The animals received swimming training for 1 week. This 1-week training program started with a duration of 10 minutes per session. Each day, the swimming time was increased by 5 minutes, gradually progressing throughout the week. By the end of the week, the rats were swimming for a total of 30 minutes per session. Then, 2 female rats and 1 male rat were put together for mating. After 48 hours, with the observance of pregnant rats, the first day of pregnancy was confirmed (15). The rats were divided into 9 groups, with each group consisting of 8 rats. Within each group, the rats were further placed in cages with 4 rats per cage. The groups are as follows: 1) control, 2) solvent, 3) cadmium, 4) silymarin, 5) training, 6) silymarin + cadmium, 7) training + cadmium, 8) training + silymarin, and 9) training + silymarin + cadmium groups.
Cadmium consumption
Cadmium chloride solvent at a dose of 400 mg/kg body weight per liter was fed to the rats through drinking a water solution (16). Therefore, 2 g of cadmium was dissolved in 5 L of water, poured into water-specific containers, and given to the rats 24 hours a day.
Procedure of silymarin consumption
To provide silymarin, first, 1.6 g of silymarin powder was dissolved in 4 mL of ethanol, and then 16 mL of distilled water was added to it. The dose of silymarin administered to the rats during pregnancy was 100 mg per kilogram of body weight. This dosage was administered by subcutaneous injection 4 days a week.
Exercise program
The duration of water training on the first day was 30 minutes. The duration was increased by 5 minutes per session, leading to a total of 60 minutes by the second week. After reaching this duration, the training time was maintained and stabilized. The exercise overload was done by adjusting the power and speed of the water while swimming (16).
Laboratory sampling and analysis
Tissue sampling was performed on the tissues of all neonatal rats with very similar conditions and in basic conditions (2 days after birth). For this purpose, a two-day-old baby was randomly selected from each mother and beheaded without anesthesia. Then, tissue samples were taken from all neonates immediately. Lung tissue was weighed using a sartorius scale: BI 1500 with an accuracy of 0.001. After that, it was put in special microtubes, immediately placed in nitrogen liquid, and kept at -80 °C until homogenization. The vascular endothelial growth factor (VEGF) level of kidney tissue was determined by enzyme-linked immunosorbent assay (ELISA) using a special kit. For this purpose, the tissues were first pulverized and then centrifuged in a homogenized buffer solution. The buffer solution used was a phosphate buffer with a pH of 7.4 and a concentration of 100mM. Additionally, the buffer solution contained 150 KIU/mL of aprotinin, which served as an antiprotease. The centrifugation process was performed for 10 minutes at a speed of 8000g. The obtained solution was transferred to the laboratory to measure the desired index using dry ice. The right kidney tissues of the animals were placed in a 10% formalin fixative solution for fixation. To prepare microscopic sections from the samples, tissue sections were prepared in the usual way. The structure of kidney tissue in different groups was presented and determined using the prepared micrographs. This study was conducted in the laboratory of the Pasteur Institute of Amol City, Iran.
Statistical methods and data analysis
To analyze the data, Kolmogorov-Smirnov tests were used to determine the normal distribution of data. A 1-way analysis of variance (ANOVA) and Tukey post hoc test were used to compare different groups. All calculations were performed using SPSS version 18 (SPSS Inc, Chicago, IL, USA). P values less than or equal to 0.05 were considered statistically significant.
Results
The results of 1-way ANOVA of PLGF showed a significant difference between the groups (F1,71 = 5.94; P ≤ 0.00). There was no significant difference between the PLGF changes in the lungs of rats in the training group compared to the control group (P < 0.162). Moreover, a significant difference was observed between PLGF changes in the lungs of rats in the cadmium group compared to the control group (P < 0.001). There was no significant difference between the PLGF changes in the lungs of rats in the cadmium group compared with the control and solvent groups .The results of statistical tests showed that there was no difference between the changes in the PLGF levels of pregnant rats in the training and cadmium groups compared to the control and cadmium groups (P ≤ 0.461), while there was a significant difference between the changes in the PLGF levels of the rats in the training and cadmium groups compared to the cadmium group (P ≤ 0.001).
Table 1 shows the one-way analysis of variance of PLGF (in terms of nanomoles per milligram of protein) in lung tissue in different research groups.
Discussion
According to the findings of the present study, after exposing pregnant rats to cadmium, a significant decrease was observed in the PLGF levels of lung tissue in the neonates of the cadmium group compared to the control and solvent groups. Increased exposure to cadmium increases ROS production. Since changes in PLGF also depend on VEGF levels, it appears that ROS stimulation may increase vascular permeability through the upregulation of PLGF. Reactive oxygen species (ROS) cause a secondary message in the function of different cells and can stimulate growth factors in different types of cells (17). Accordingly, cadmium is expected to increase PLGF levels by creating OS, which is contrary to the results obtained in this study. The decrease in this factor due to cadmium can be attributed to the dose of cadmium consumed. Therefore, it can be suggested that cadmium with this dose causes the destruction of lung tissue and thus reduces PLGF levels.
In the present study, after subcutaneous injection of silymarin, a significant increase was observed in PLGF levels of lung tissue in the neonates of the silymarin group compared to the control and solvent groups. Since there is little information on the effect of silymarin supplementation on PLGF levels, it will be very difficult to discuss this issue. The PLGF growth factor is the second factor in the VEGF family, which, like other members of the VEGF family, has different isoforms that are mainly expressed in pairs during all periods of pregnancy. This study demonstrates that PLGF is necessary for the development of vascular and physiological factors. Research has shown that its functions depend on the growth stages and physiological function of the organ in which it is secreted (18). Accordingly, silymarin is expected to reduce PLGF levels due to its antiangiogenic properties, which is contrary to the results obtained in this study. This discrepancy may be attributed to the critical stage of pregnancy and fetal development. Since a study has shown the effect of silymarin on wound healing in diabetic rats (19), it can be concluded that if silymarin is used in physiological conditions where PLGF levels are high, it causes a further increase in PLGF levels, and in pathological conditions, where PLGF levels are high, silymarin exerts its anti-angiogenic properties, which causes a decrease in PLGF levels. The antiangiogenic activity of silymarin is one of the main ways to treat cancer, especially in the case of solid tumors (20). This silymarin activity has been well investigated in umbilical vein endothelial cells, where silybin activity is dose-dependent and VEGF reduction has been reported. This study showed a significant increase in the PLGF levels of lung tissue in the neonates of the exercise group compared to the control and solvent groups. Studies have shown that PLGF is a key factor in the development of various angiogenic adaptations after endurance training. In addition, endurance training increases PLGF activity (21,22). In contrast, Ferland et al (2013) examined the relationship between physical activity and fetal growth factor levels during pregnancy and found that with increasing levels of physical activity, PLGF concentration decreased significantly (23).
It seems that different factors must work together locally and systematically to make adaptations through endurance training. Local hypoxia due to endurance training is one of the most basic stimuli for adaptations, such as increased capillary density and oxidative capacity (23). Mechanisms that are sensitive to oxygen stress can adapt to changes as a result of endurance training (24). Due to oxidative damage to lipids, products (such as malondialdehyde) are produced, which are used as an indicator of fat damage. Therefore, it seems that intense exercise can be harmful and increase lipid peroxidation (23). Accordingly, an increase in PLGF in neonatal lung tissue after 3 weeks of endurance swimming may be related to OS due to this activity and the production of ROS. Increased PLGF in the lung tissue of infants who have not been directly involved in any exercise program indicates exercise adaptation. It is likely that exercise during pregnancy is effective in increasing neonatal endurance. Therefore, due to the needs of the fetus in a hypoxic environment for the development of fetal vessels, exercise may be able to help the process of fetal growth and development by creating such an environment.
In this study, cadmium alone caused a significant decrease in PLGF levels in neonatal lung tissue compared to the control group, confirming tissue changes following cadmium consumption. Therefore, it can be suggested that cadmium with this dose causes the destruction of lung tissue and thus reduces PLGF levels. In other groups (including the cadmium group), a significant increase was observed in PLGF levels in neonatal lung tissue compared to the control and cadmium groups, indicating that the effect of cadmium was neutralized by silymarin and exercise alone or in combination. Exercise was more effective than silymarin in compensating for the decrease in PLGF levels. Melikoglu et al (2008) showed that a regular long-term exercise period could increase many antioxidant enzymes, especially glutathione, which can counteract cadmium toxicity for the reasons mentioned in the silymarin section (25).
Based on the findings of this study, it can be said that 3 weeks of submaximal endurance swimming training during pregnancy can prevent the destruction of PLGF-dependent growth processes during the fetal period by compensating for the reduction of PLGF levels caused by cadmium pollutants, as well as can be seen as a strategy for moderating the destructive effects of cadmium pollutants. The injection of 400 mg of silymarin per kilogram of body weight caused a significant increase in PLGF levels in neonatal lung tissues compared to the control and solvent groups. Meanwhile, the silymarin solvent group, which received the same amount of alcohol and distilled water, did not show any significant changes in PLGF levels, indicating that this solvent did not affect the results of the study. In the silymarin + exercise group, a significant increase was observed in PLGF levels compared to the silymarin group. Thus, exercise with silymarin could have a greater effect on increasing PLGF levels, but PLGF levels decreased in the silymarin + cadmium group compared to the silymarin group, which was not statistically significant. In other words, cadmium could not exert its effect in the presence of silymarin.
This study demonstrated that silymarin can be classified as a flavonoid that causes positive regulation of PLGF in pregnancy. Silymarin has been shown to increase hepatic glutathione by 35% in healthy individuals. This effect has also been observed in gastric and intestinal cells (26). Rajswara et al (2007) also showed that silymarin increased the number of endogenous antioxidant enzymes, such as glutathione transferase (27). This effect of silymarin on the liver can probably be extended to kidney tissue. Low glutathione is directly related to an increase in cadmium-induced apoptosis; thus, a silymarin-induced glutathione increase can be a rational explanation for combating cadmium toxicity (28). As a result, it can be stated that silymarin mitigates the negative effects of cadmium on fetal growth by compensating for the decrease in PLGF levels caused by this pollutant and could be used as a compound to help fetal growth in mothers exposed to environmental pollutants. The findings of the present study showed that exercise alone significantly increased PLGF levels in neonatal lung tissue compared to the control group.
Conclusion
Cadmium caused a significant decrease in PLGF levels in neonatal lung tissue. In addition, the use of regular swimming endurance exercises and silymarin supplementation prevented the effects of cadmium chloride. Therefore, it seems that the protective effects of regular and submaximal swimming endurance exercises and silymarin supplementation during pregnancy can be used as a useful and effective strategy against the effects and pollution caused by cadmium. Since regular aerobic activity and the use of herbal antioxidant supplements as a non-pharmacological strategy lead to the protective effects of the body’s vascular system during pregnancy, the effectiveness of the combined strategy is far greater than the individual effects of each of these approaches.
Acknowledgement
The author would like to express gratitude to the professors and colleagues who have worked together in carrying out this research.
Funding sources
In this project, no person or organization has cooperated financially or technically.
Ethical statement
All stages of practice and research implementation according to Institute of Health and Nutrition Guidelines on Care and Use of Laboratory Animals and Faculty Ethics Committee Physical education and sports sciences of Mazandaran University were carried out. All the activities of ethics in research in the years before 1400 were legally with Mazandaran University Institute itself.
Conflicts of interest
The authors declare that there is no conflict of interest.
Author contributions
This article has been derived from a thesis approved by the Islamic Azad University of Sari. The authors gratefully acknowledge cooperation in the study.
Pregnancy is a stressful condition in which many physiological and metabolic functions change considerably (1). In a healthy person, free radicals and antioxidants are in balance, but when this balance is disrupted, oxidative stress (OS) occurs. This OS causes tissue damage and various diseases (2). Oxidative stress plays a role in the development of health conditions, such as preterm delivery, fetal growth restriction, pregnancy poisoning, and abortion (3). Cadmium is one of the main environmental pollutants, having many effects on the surrounding environment, and even a small amount of it is very poisonous and causes cancer (4). This element has been reported to be associated with the occurrence of OS, causing reactive oxygen species (ROS) directly and indirectly. In addition, the accumulation of cadmium-induced free radicals can damage the structure and performance of different cells (including beta cells) and may result in pancreatic cancer (5,6). When cadmium passes the placenta and enters the fetus’s body, it causes different abnormalities, including cleft palate, cleft lip, non-formation of the ear or misplaced ear, and open eye (7,8). The proper use of dietary, medicinal, and herbal supplements can be helpful in minimizing the effect of cadmium. Silybum marianum is one of the vegetables that are rich in phenolic flavonolignan compounds (taxifolin, silibinin, silidianin, and silicristin) (9). The most important uses of this plant include its antioxidant, anti-inflammatory, and anticancer properties, as well as its effect on plasma lipids and lipoproteins. However, research on the inhibitory effects of Silibium marianum in contrast to the effects of exercise and physical activity on fetal growth factor is still unknown.
Placental growth factor (PLGF) is considered one of the strong angiogenic factors in tumors (10), which has been identified in inflammatory diseases, wound healing, and cancers. It is also involved in cardiovascular diseases, monocyte invasion in chronic infectious diseases, and increased internal thickness of atherosclerosis (11). Abnormal concentrations of this factor cause abnormal placental development that is observed during preeclampsia in pregnancy (12). The presence of this factor in early life after birth is essential for the growth and survival of infants, and its prevention leads to increased mortality, stunted growth, and impaired organ development (12). Angiogenesis is a process that develops in a complex way during life and adulthood. Physical exercise has been identified as an effective factor in promoting angiogenesis (13). However, few studies have been conducted on the effects of sports activities, especially swimming, on the PLGF of pregnant women who had moderate and regular aerobic exercise compared to the inactive group (14). Since it has been suggested that cadmium has an effect on the function of various cells, including endothelial cells of blood vessels and beta cells, it is possible that it has an effect on PLGF levels as well. Therefore, it is possible to use supplements
Since cadmium has been suggested to affect the function of various cells, it has been suggested that cadmium has an effect on the function of various cells, including endothelial cells of blood vessels and beta cells, it is possible that it has an effect on PLGF levels as well. Therefore, the use of supplements such as silymarin may be an effective factor in reducing the risks and diseases caused by the effect of cadmium on PLGF.
To date, no study has been reported to investigate the effect of submaximal swimming activity on PLGF concentration during pregnancy, as well as the effect of silymarin and cadmium supplementation on PLGF levels.
Methods
Seventy-two 8-week-old pregnant Wistar rats (weighing 20 ± 200 g) were divided into 9 groups, with 8 rats in each group. The rats were kept in transparent polycarbonate cages at a temperature of 23 ± 2 °C with a humidity of 45% ± 5% and light-dark cycle of 12:12. During the research period, all animals were provided ad libitum access to standard food pellets and water. One week after transportation to the laboratory and adaptation to the new environment, the animals were transferred to a swimming pool. This was done to help the rats become familiar with the water, reduce swimming-related stress, and provide them with a training environment. The animals received swimming training for 1 week. This 1-week training program started with a duration of 10 minutes per session. Each day, the swimming time was increased by 5 minutes, gradually progressing throughout the week. By the end of the week, the rats were swimming for a total of 30 minutes per session. Then, 2 female rats and 1 male rat were put together for mating. After 48 hours, with the observance of pregnant rats, the first day of pregnancy was confirmed (15). The rats were divided into 9 groups, with each group consisting of 8 rats. Within each group, the rats were further placed in cages with 4 rats per cage. The groups are as follows: 1) control, 2) solvent, 3) cadmium, 4) silymarin, 5) training, 6) silymarin + cadmium, 7) training + cadmium, 8) training + silymarin, and 9) training + silymarin + cadmium groups.
Cadmium consumption
Cadmium chloride solvent at a dose of 400 mg/kg body weight per liter was fed to the rats through drinking a water solution (16). Therefore, 2 g of cadmium was dissolved in 5 L of water, poured into water-specific containers, and given to the rats 24 hours a day.
Procedure of silymarin consumption
To provide silymarin, first, 1.6 g of silymarin powder was dissolved in 4 mL of ethanol, and then 16 mL of distilled water was added to it. The dose of silymarin administered to the rats during pregnancy was 100 mg per kilogram of body weight. This dosage was administered by subcutaneous injection 4 days a week.
Exercise program
The duration of water training on the first day was 30 minutes. The duration was increased by 5 minutes per session, leading to a total of 60 minutes by the second week. After reaching this duration, the training time was maintained and stabilized. The exercise overload was done by adjusting the power and speed of the water while swimming (16).
Laboratory sampling and analysis
Tissue sampling was performed on the tissues of all neonatal rats with very similar conditions and in basic conditions (2 days after birth). For this purpose, a two-day-old baby was randomly selected from each mother and beheaded without anesthesia. Then, tissue samples were taken from all neonates immediately. Lung tissue was weighed using a sartorius scale: BI 1500 with an accuracy of 0.001. After that, it was put in special microtubes, immediately placed in nitrogen liquid, and kept at -80 °C until homogenization. The vascular endothelial growth factor (VEGF) level of kidney tissue was determined by enzyme-linked immunosorbent assay (ELISA) using a special kit. For this purpose, the tissues were first pulverized and then centrifuged in a homogenized buffer solution. The buffer solution used was a phosphate buffer with a pH of 7.4 and a concentration of 100mM. Additionally, the buffer solution contained 150 KIU/mL of aprotinin, which served as an antiprotease. The centrifugation process was performed for 10 minutes at a speed of 8000g. The obtained solution was transferred to the laboratory to measure the desired index using dry ice. The right kidney tissues of the animals were placed in a 10% formalin fixative solution for fixation. To prepare microscopic sections from the samples, tissue sections were prepared in the usual way. The structure of kidney tissue in different groups was presented and determined using the prepared micrographs. This study was conducted in the laboratory of the Pasteur Institute of Amol City, Iran.
Statistical methods and data analysis
To analyze the data, Kolmogorov-Smirnov tests were used to determine the normal distribution of data. A 1-way analysis of variance (ANOVA) and Tukey post hoc test were used to compare different groups. All calculations were performed using SPSS version 18 (SPSS Inc, Chicago, IL, USA). P values less than or equal to 0.05 were considered statistically significant.
Results
The results of 1-way ANOVA of PLGF showed a significant difference between the groups (F1,71 = 5.94; P ≤ 0.00). There was no significant difference between the PLGF changes in the lungs of rats in the training group compared to the control group (P < 0.162). Moreover, a significant difference was observed between PLGF changes in the lungs of rats in the cadmium group compared to the control group (P < 0.001). There was no significant difference between the PLGF changes in the lungs of rats in the cadmium group compared with the control and solvent groups .The results of statistical tests showed that there was no difference between the changes in the PLGF levels of pregnant rats in the training and cadmium groups compared to the control and cadmium groups (P ≤ 0.461), while there was a significant difference between the changes in the PLGF levels of the rats in the training and cadmium groups compared to the cadmium group (P ≤ 0.001).
Table 1 shows the one-way analysis of variance of PLGF (in terms of nanomoles per milligram of protein) in lung tissue in different research groups.
Table 1. Results of 1-way analysis of variance of PLGF (placental growth factor) for groups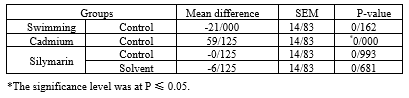 |
Discussion
According to the findings of the present study, after exposing pregnant rats to cadmium, a significant decrease was observed in the PLGF levels of lung tissue in the neonates of the cadmium group compared to the control and solvent groups. Increased exposure to cadmium increases ROS production. Since changes in PLGF also depend on VEGF levels, it appears that ROS stimulation may increase vascular permeability through the upregulation of PLGF. Reactive oxygen species (ROS) cause a secondary message in the function of different cells and can stimulate growth factors in different types of cells (17). Accordingly, cadmium is expected to increase PLGF levels by creating OS, which is contrary to the results obtained in this study. The decrease in this factor due to cadmium can be attributed to the dose of cadmium consumed. Therefore, it can be suggested that cadmium with this dose causes the destruction of lung tissue and thus reduces PLGF levels.
In the present study, after subcutaneous injection of silymarin, a significant increase was observed in PLGF levels of lung tissue in the neonates of the silymarin group compared to the control and solvent groups. Since there is little information on the effect of silymarin supplementation on PLGF levels, it will be very difficult to discuss this issue. The PLGF growth factor is the second factor in the VEGF family, which, like other members of the VEGF family, has different isoforms that are mainly expressed in pairs during all periods of pregnancy. This study demonstrates that PLGF is necessary for the development of vascular and physiological factors. Research has shown that its functions depend on the growth stages and physiological function of the organ in which it is secreted (18). Accordingly, silymarin is expected to reduce PLGF levels due to its antiangiogenic properties, which is contrary to the results obtained in this study. This discrepancy may be attributed to the critical stage of pregnancy and fetal development. Since a study has shown the effect of silymarin on wound healing in diabetic rats (19), it can be concluded that if silymarin is used in physiological conditions where PLGF levels are high, it causes a further increase in PLGF levels, and in pathological conditions, where PLGF levels are high, silymarin exerts its anti-angiogenic properties, which causes a decrease in PLGF levels. The antiangiogenic activity of silymarin is one of the main ways to treat cancer, especially in the case of solid tumors (20). This silymarin activity has been well investigated in umbilical vein endothelial cells, where silybin activity is dose-dependent and VEGF reduction has been reported. This study showed a significant increase in the PLGF levels of lung tissue in the neonates of the exercise group compared to the control and solvent groups. Studies have shown that PLGF is a key factor in the development of various angiogenic adaptations after endurance training. In addition, endurance training increases PLGF activity (21,22). In contrast, Ferland et al (2013) examined the relationship between physical activity and fetal growth factor levels during pregnancy and found that with increasing levels of physical activity, PLGF concentration decreased significantly (23).
It seems that different factors must work together locally and systematically to make adaptations through endurance training. Local hypoxia due to endurance training is one of the most basic stimuli for adaptations, such as increased capillary density and oxidative capacity (23). Mechanisms that are sensitive to oxygen stress can adapt to changes as a result of endurance training (24). Due to oxidative damage to lipids, products (such as malondialdehyde) are produced, which are used as an indicator of fat damage. Therefore, it seems that intense exercise can be harmful and increase lipid peroxidation (23). Accordingly, an increase in PLGF in neonatal lung tissue after 3 weeks of endurance swimming may be related to OS due to this activity and the production of ROS. Increased PLGF in the lung tissue of infants who have not been directly involved in any exercise program indicates exercise adaptation. It is likely that exercise during pregnancy is effective in increasing neonatal endurance. Therefore, due to the needs of the fetus in a hypoxic environment for the development of fetal vessels, exercise may be able to help the process of fetal growth and development by creating such an environment.
In this study, cadmium alone caused a significant decrease in PLGF levels in neonatal lung tissue compared to the control group, confirming tissue changes following cadmium consumption. Therefore, it can be suggested that cadmium with this dose causes the destruction of lung tissue and thus reduces PLGF levels. In other groups (including the cadmium group), a significant increase was observed in PLGF levels in neonatal lung tissue compared to the control and cadmium groups, indicating that the effect of cadmium was neutralized by silymarin and exercise alone or in combination. Exercise was more effective than silymarin in compensating for the decrease in PLGF levels. Melikoglu et al (2008) showed that a regular long-term exercise period could increase many antioxidant enzymes, especially glutathione, which can counteract cadmium toxicity for the reasons mentioned in the silymarin section (25).
Based on the findings of this study, it can be said that 3 weeks of submaximal endurance swimming training during pregnancy can prevent the destruction of PLGF-dependent growth processes during the fetal period by compensating for the reduction of PLGF levels caused by cadmium pollutants, as well as can be seen as a strategy for moderating the destructive effects of cadmium pollutants. The injection of 400 mg of silymarin per kilogram of body weight caused a significant increase in PLGF levels in neonatal lung tissues compared to the control and solvent groups. Meanwhile, the silymarin solvent group, which received the same amount of alcohol and distilled water, did not show any significant changes in PLGF levels, indicating that this solvent did not affect the results of the study. In the silymarin + exercise group, a significant increase was observed in PLGF levels compared to the silymarin group. Thus, exercise with silymarin could have a greater effect on increasing PLGF levels, but PLGF levels decreased in the silymarin + cadmium group compared to the silymarin group, which was not statistically significant. In other words, cadmium could not exert its effect in the presence of silymarin.
This study demonstrated that silymarin can be classified as a flavonoid that causes positive regulation of PLGF in pregnancy. Silymarin has been shown to increase hepatic glutathione by 35% in healthy individuals. This effect has also been observed in gastric and intestinal cells (26). Rajswara et al (2007) also showed that silymarin increased the number of endogenous antioxidant enzymes, such as glutathione transferase (27). This effect of silymarin on the liver can probably be extended to kidney tissue. Low glutathione is directly related to an increase in cadmium-induced apoptosis; thus, a silymarin-induced glutathione increase can be a rational explanation for combating cadmium toxicity (28). As a result, it can be stated that silymarin mitigates the negative effects of cadmium on fetal growth by compensating for the decrease in PLGF levels caused by this pollutant and could be used as a compound to help fetal growth in mothers exposed to environmental pollutants. The findings of the present study showed that exercise alone significantly increased PLGF levels in neonatal lung tissue compared to the control group.
Conclusion
Cadmium caused a significant decrease in PLGF levels in neonatal lung tissue. In addition, the use of regular swimming endurance exercises and silymarin supplementation prevented the effects of cadmium chloride. Therefore, it seems that the protective effects of regular and submaximal swimming endurance exercises and silymarin supplementation during pregnancy can be used as a useful and effective strategy against the effects and pollution caused by cadmium. Since regular aerobic activity and the use of herbal antioxidant supplements as a non-pharmacological strategy lead to the protective effects of the body’s vascular system during pregnancy, the effectiveness of the combined strategy is far greater than the individual effects of each of these approaches.
Acknowledgement
The author would like to express gratitude to the professors and colleagues who have worked together in carrying out this research.
Funding sources
In this project, no person or organization has cooperated financially or technically.
Ethical statement
All stages of practice and research implementation according to Institute of Health and Nutrition Guidelines on Care and Use of Laboratory Animals and Faculty Ethics Committee Physical education and sports sciences of Mazandaran University were carried out. All the activities of ethics in research in the years before 1400 were legally with Mazandaran University Institute itself.
Conflicts of interest
The authors declare that there is no conflict of interest.
Author contributions
This article has been derived from a thesis approved by the Islamic Azad University of Sari. The authors gratefully acknowledge cooperation in the study.
Research Article: Original Paper |
Subject:
Sport Physiology
Received: 2021/09/19 | Accepted: 2022/08/3 | Published: 2024/01/15 | ePublished: 2024/01/15
Received: 2021/09/19 | Accepted: 2022/08/3 | Published: 2024/01/15 | ePublished: 2024/01/15
References
1. Demirevska-Kepova K, Simova-Stoilova L, Stoyanova ZP, Feller U. Cadmium stress in barley: growth, leaf pigment, and protein composition and detoxification of reactive oxygen species. Journal of plant nutrition. 2006; 29(3): 451-68. [View at Publisher] [DOI] [Google Scholar]
2. Dai S, Yin Z, Yuan G, Lu H, Jia R, Xu J, et al., Quantification of metallothionein on the liver and kidney of rats by subchronic lead and cadmium in combination. Environ toxicol pharmacol. 2013; 36(3): 1207-16. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Hsu CW, Lin JL, Lin-Tan DT, Huang WH, Chen KH, Yen TH. Association between blood cadmium levels and malnutrition in peritoneal dialysis. BMC Nephrol. 2014;15:1-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Kim J, Lim W, Ko Y, Kwon H, Kim S, Kim O, et al. The effects of cadmium on VEGF‐mediated angiogenesis in HUVECs. J Appl Toxicol. 2012;32(5):342-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Dover N, Gulerman HC, Celen S, Kahyaoglu S, Yenicesu O. Placental growth factor: as an early second trimester predictive marker for preeclampsia in normal and high-risk pregnancies in a Turkish population. J Obstet Gynecol India. 2013;63(3):158-63. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Adu-Bonsaffoh K, Ansong Antwi D, Gyan B, Amenyi Obed S. Endothelial dysfunction in the pathogenesis of pre-eclampsia in Ghanaian women. BMC physiol. 2017;17(1):1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Trivedi D, Cochrane review summary: specialised antenatal clinics for women with a multiple pregnancy for improving maternal and infant outcomes. Prim Health Care Res Dev. 2014;15(1):3-4. [View at Publisher] [DOI3] [PMID] [Google Scholar]
8. Toescu V, Nuttall SL, Martin U, Nightingale P, Kendall MJ, Brydon P, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci. 2004;106(1):93-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Radko L, Cybulski W. Application of silymarin in human and animal medicine. Journal of Pre-Clinical and Clinical Research. 2007;1(1):22-6. [View at Publisher] [Google Scholar]
10. Lee B, Choi GM, Sur B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: the possible role of serotonin. BMC complement Med Ther. 2020;20(1):1-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Aref S, H Goda, E Abdelaal, Circulating vascular growth factor (VEGF) Angiopoietin-1 (Angi-1) and soluble Tie-2 receptor in pregnancy complicated with pre-eclampsia: a prospective study. J Obstet Gynaecol India. 2013;63(5):316-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Korsak J, Rzeszotarska A, Marczyński W, Jabłońska I, Białecki J, Walczak P. Concentration of platelet derived-growth factors in concentrates used to regenerate injured bone tissue. Ortop Traumatol Rehabil. 2013;15(5):379-88. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Gigante B, Tarsitano M, Cimini V, De Falco S, Persico MG. Placenta growth factor is not required for exercise-induced angiogenesis. Angiogenesis. 2004; 7(3): 277-284. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Masuda E, Sista AK, Pua BB, Madoff DC. Palliative procedures in lung cancer. Semin Intervent Radiol. 2013;30(2):199-205. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Bhattacharjee J, Mohammad S, Goudreau AD, Adamo KB. Physical activity differentially regulates VEGF, PlGF, and their receptors in the human placenta. Physiol Rep. 2021;9(2):e14710. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Koumentaki A, Anthony F, Poston L, Wheeler T. Low-protein diet impairs vascular relaxation in virgin and pregnant rats. Clin Sci. 2002;102(5):553-60. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Mirdar S, Arab A, Hedayati M, Hajizade A. Evaluation of the Effect of a Swimming Training Program on Levels of Lung Hypoxia Inducible Factor-1α (HIF-1α) in Pups. Qom Univ Med Sci J. 2013;7(3):11-20. [View at Publisher] [Google Scholar]
18. Al-Saleh I, Coate L. Cadmium exposure in Saudi Arabia and its relationship to smoking. Trace elements in medicine. 1993;10(3):129-33. [View at Publisher] [Google Scholar]
19. dos Reis Veloso CE, Frota de Almeida LN, Recchia FM, Pelayes D, Nehemy MB. VEGF gene polymorphism and response to intravitreal ranibizumab in neovascular age-related macular degeneration. Ophthalmic Res. 2014;51(1):1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. El Sherif F, Khattab S, Ibrahim AK, Ahmed SA. Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiol Mol Biol Plants. 2013;19(1):127-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies-a review. J Adv Res. 2017;8(6):591-605. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Weissgerber TL, Davies GAL, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. J Appl Physiol. 2010;108(5):1217-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Clapp III JF, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000;183(6):1484-1488. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clin Sci. 2006;111(6):401-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Melikoglu M, Kaldirimci M, Katkat D, Sen I, Kaplan I, Senel K. The effect of regular long term training on antioxidant enzymatic activities. J Sports Med Phys Fitness. 2008;48(3):388-90. [View at Publisher] [Google Scholar]
27. Panjehpour M, Bayesteh M. The cytotoxic effects of cadmium chloride on the human lung carcinoma (Calu-6) cell line. Research in Pharmaceutical Sciences. 2009;3(2):49-53. [View at Publisher] [Google Scholar]
28. Rao PR, Viswanath RK. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp Clin Cardiol. 2007;12(4):179-87. [View at Publisher] [Google Scholar]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com