Volume 17, Issue 6 (Nov-Dec 2023)
mljgoums 2023, 17(6): 16-18 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
fatemi M, Ghandehari F, Salehi D, Torabian P. Comparing the cytotoxic effects of iron oxide nanoparticles synthesized using the cytoplasmic extract of Lactobacillus casei and chemical synthesis. mljgoums 2023; 17 (6) :16-18
URL: http://mlj.goums.ac.ir/article-1-1410-en.html
URL: http://mlj.goums.ac.ir/article-1-1410-en.html
1- Department of Biology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
2- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran ,fe_gh_2010@yahoo.com
3- Department of Chemical Engineering, Isfahan University of Technology, Isfahan, Iran
4- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
2- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran ,
3- Department of Chemical Engineering, Isfahan University of Technology, Isfahan, Iran
4- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
Full-Text [PDF 510 kb]
(726 Downloads)
| Abstract (HTML) (2854 Views)



Discussion
Cancer is the leading reason of mortality in developed countries and the second leading cause of death in developing countries. Despite many efforts in the prevention and treatment of cancer, the disease has grown exponentially. It is also considered a global killer. Despite the common treatments for cancer, one of the ways to improve the effects of chemotherapy drugs is to use compounds that are among the natural products that can reduce the side effects and have a strong effect on cancer cells. Reducing drug doses and providing greater efficiency in tumor diagnosis, tumor targeting and treatment, biocompatibility, and biodegradability are among the factors that make Fe2o3 NPs a suitable candidate for clinical applications, such as cell therapy, tissue repair, and drug guidance (10).
Today, the production of biological NPs is increasing due to their efficiency in medicine and life sciences. On the other hand, increasing awareness of green chemistry and biological processes has necessitated using environmentally friendly methods for the non-toxic production of biological nanomaterials. Although various biological pathways for the biological production of metal NPs are known, using plants or other intermediates to produce metal NPs is expensive and limited. Therefore, easy bio-preparation of NPs with size and shape control is of great importance in the proposed methods. In the meantime, using a microorganism that can stably produce NPs outside the cell, with a suitable particle size distribution, is an environmentally safe and interesting method due to its lower cost (11,12). Numerous studies have investigated the biosynthesis of NPs. Ghandehari et al showed the great potential of L. fermentum in the production of Fe2o3 NPs with a size of 20 nm (6). Comparing the results of this study with other studies showed that the chemical characteristics of Fe2o3 NPs taken from the cytoplasmic extract of L. casei are consistent with other studies. Previous studies have shown that microorganisms are able to synthesize metal NPs in the size of 1 to 200 nm using inexpensive and renewable reducing agents (13-15). The cytoplasmic extract of this bacterium seems to contain lactate or acetate-reducing agents, decreasing metal iron to Fe2o3 NPs. Many studies have shown that proteins and enzymes in the cytoplasmic extract of microorganisms play an important role in the synthesis of NPs (16, 17).
Comparing the cytotoxic effects of green and chemically synthesized Fe2o3 NPs showed that the synthesized green and chemical NPs with increasing concentrations revealed a great lethal impact on cancer cells, and synthesized green NPs at higher concentrations with a significant difference compared to chemical NPs reduced the survival of cancer cells. Nevertheless, the cytotoxic effects of NPs on normal cells also increased by increasing the concentration of NPs. The synthesized NPs manifested less cytotoxic effects on normal cells at a 100-μg/mL concentration; thus, it is suggested that this concentration can be used to inhibit the growth of cancer cells. Additional investigations have explained that the cytotoxic effects of NPs are concentration-dependent, bioparticles give more inhibitory effects on cancer cells compared to chemical NPs, and there is a direct relationship between NP concentration and NP toxicity on the cell lines tested. These results are perfectly consistent with this research (18-20). DNA damage is the most important cause of cancer cells, and this damage to genes can make a big difference between cancer cells and normal cells. These differences can also be used to recognize and destroy cancer cells accurately.
The mechanism of NP toxicity is not yet well understood; however, studies suggest that NPs play a key role in DNA damage, membrane destruction, and, ultimately, cell death by creating oxidative stress and lipid peroxidation, which can lead to effective therapies (21). Also, due to the high activity of mitochondria in the process of respiration of cancer cells compared to normal cells, a suitable substrate is provided for NPs to destroy cancer cells. Differences in the shape, size, and surface charge of NPs are other factors that contribute to differences in the toxicity of NPs between cancer cells and normal cells (22(. Therefore, Fe2o3 NPs synthesized by green and chemical methods can be used as a suitable solution in cancer treatment. Since green synthesis is an environmentally friendly method, it is recommended to use biological compounds to synthesize NPs to fight cancer.
Conclusion
Synthesizing Fe2o3 NPs by the cytoplasmic extract of L. casei was successful and had favorable cytotoxic effects on MCF-7 cancer cells. The findings were more significant than chemically synthesized NPs. The synthesized green NPs presented the most insignificant toxic effect on normal HEK-293 cells at low concentrations compared to chemically synthesized NPs. It seems that green synthesis can be regarded in cytotoxic investigations.
Acknowledgement
We gratefully acknowledge the assistance of Islamic Azad University of Flavarjan and the Department of Microbiology for their generous support, providing us with the necessary laboratories and equipment to conduct this study.
Funding sources
N/A.
Ethical statement
This study was approved by the Ethics Committee of Islamic Azad University, Falavarjan Branch, Isfahan, Iran.
Conflicts of interest
There is no conflict of interest.
Author contributions
This article was extracted from a master's thesis project in microbiology conducted by Dr Ghandhari, Dr Fatemi, Salehi, and Torabian at Islamic Azad University, Flavarjan Branch, Flavarjan, Isfahan, Iran.
Full-Text: (588 Views)
Introduction
Cancer is a malignant disease with a significant mortality rate that leads to numerous psychological and economic challenges. Notwithstanding numerous treatments (such as chemotherapy, immunotherapy, and radiotherapy), cancer is still a significant challenge. Consequently, developing suitable, biocompatible, low-cost cancer treatment approaches is essential (1). It is proven that NPs can be used as a therapeutic agent in addition to fighting bacteria and viruses in cancer. Iron oxide nanoparticles (Fe2o3 NPs) have a broader scope of biomedical applications than other magnetic NPs. These NPs are extremely significant in medicine due to their biocompatibility with environmental conditions, excellent magnetic properties, stability, and easy preparation. However, the organic solvents used to produce these NPs are toxic and can have destructive environmental impacts. Accordingly, there is a strong tendency to use reliable methods to synthesize NPs (2).
Nanobiotechnology was applied to biological systems (such as bacteria, fungi, and plants) to produce NPs. Presently, Fe2o3 NPs are produced using the “green synthesis method.” This method used biological sources, including bacteria, green algae, fungi, actinomycetes, and plant extracts (3). Green synthesis is clean, simple, eco-friendly, and non-toxic. On the other hand, the pathogenic effect of some organisms has been reported (4).
This study used Lactobacillus casei (PTCC: 1608). It is a rod, gram-positive organism known as a probiotic bacterium. Probiotics are defined as live microorganisms that, when consumed in sufficient quantities, provide a health benefit to the host (5).
Then, the cytotoxic effects of the synthesized Fe2o3 NPs were investigated on MCF-7 and human embryonic kidney 293 (HEK-293) cells as cancerous and normal cells, respectively.
Methods
Lactobacillus casei with (PTCC: 1608) identification was obtained from the microbial bank of the Iranian Research Organization for Science and Industry. The cytoplasmic extract was designed by freeze-thaw method and added to a 3-10M aqueous solution of Fe2o3 )Feo). Following regulating the pH of the solution to 5.6, it was incubated in darkness for 3 weeks at 37 °C (6).
The coprecipitation method was applied to synthesize the Fe3O4 NPs. Briefly, FeSO4 and NaOH were poured into separate beakers, 100 mL of deionized water was added to both beakers, and 2 capsule-rotating magnets were thrown inside. Then, both beakers were placed on a heater equipped with a magnetic stirrer to completely dissolve the materials. In the next step, FeCl3·6H2O was added to the solution. After creating an orange color solution, a black precipitate with a nanostructure was formed by adding NaOH. Finally, the excess salts were washed, and the precipitate was dried (7).
Eventually, a desiccator pulverized the response solution, and an X-ray diffraction (XRD) device (Philips PW 1710, NY, USA) was used to investigate the production of NPs. The sample was put on a glass substrate, and the scan was conducted with angle θ2 and in the range of 20 to 80 degrees. The voltage used was 40 kV, and the current was 30 mA. The particle size was calculated by the Debye-Scherrer equation handling with the following formula: D=0.9/ ß cosØA. A transmission electron microscope (Philips 208S 100 kV, Netherlands) was used to investigate the shape and size of NPs (6).
MCF-7 breast cancer cells and normal HEK-293 cells were acquired from the Pasteur Institute of Iran. For cell culture, cell lines were incubated in RPMI-1640 culture medium (Idea Zist Company, Iran) containing 10% FBS and 1% penicillin and streptomycin (Gibco, USA) in the presence of 5% CO2 at 37 °C. The cell environment was exchanged once every 3 days continuously; the cells reached a concentration of 80% for treatment. The cytotoxic effects of Fe2o3 NPs synthesized by the cytoplasmic extract of L. casei on MCF-7 breast cancer cells and normal HEK-293 cells were investigated using the MTT method (6,8). This process is based on the activity of mitochondrial dehydrogenases, which are significant markers in survival. Thus, the cells were cultured at a density of 104 cells in each well of a 96-well plate. Following 24 hours, the cell surface environment was modified and treated with varying concentrations of 10, 100, and 1000 μg/mL of Fe2o3 NPs for 48 hours, and cytotoxicity was evaluated by the MTT method.
The cytotoxic effects of Fe3O4 NPs synthesized on MCF-7 cancer cells and HEK-293 cells were studied using the Microculture Tetrazolium Test (MTT). MTT is reduced in the mitochondria of metabolically active cells by succinate dehydrogenase to yield a water-insoluble purple formazan crystal. To this purpose, 104 cells were cultured per well in a 96-well plate. After 24 hours of incubation, cell supernatants were removed, and they were treated with different concentrations of 10, 100, and 1000 µg/mL of Fe3O4 NPs for another 48 hours. Then, the cells were washed twice with PBS and incubated with 0.5 mg/mL MTT in a culture medium for 2.5 hours in a humidified atmosphere containing 5% CO2 at 37 °C. Following incubation, the MTT solution was removed, and formazan was extracted from cells with 200 μL of DMSO. The formazan-specific light absorption was then measured at 595 nm with a spectrophotometer (Rayleigh UV-2601, China). The experiment was repeated three times (9).
The data for each group were expressed as mean ± SD. An independent t test was used to compare viability between the 2 cell lines, and a 1-way analysis of variance (ANOVA) was used to analyze the results of the cytotoxic effect of the NPs on the cell lines. All analyses were performed using SPSS version 16 (SPSS Inc, Chicago, IL, USA). The statistical significance of all data was set at P < 0.05, P < 0.01, and P < 0.001.
Results
According to the XRD analysis results, the refractive peaks corresponding to angles θ2 or equal θ = 74°, 47°, 43° and 35°which can be indexed to (440), (400), (311), and 220. These peaks corresponded to the XRD pattern of Fe2o3. The NP size was calculated by the Debay-Scherrer relationship in the green synthesis of 15 nm and the chemical synthesis of 20 nm (Figures 1A and 1B).
Cancer is a malignant disease with a significant mortality rate that leads to numerous psychological and economic challenges. Notwithstanding numerous treatments (such as chemotherapy, immunotherapy, and radiotherapy), cancer is still a significant challenge. Consequently, developing suitable, biocompatible, low-cost cancer treatment approaches is essential (1). It is proven that NPs can be used as a therapeutic agent in addition to fighting bacteria and viruses in cancer. Iron oxide nanoparticles (Fe2o3 NPs) have a broader scope of biomedical applications than other magnetic NPs. These NPs are extremely significant in medicine due to their biocompatibility with environmental conditions, excellent magnetic properties, stability, and easy preparation. However, the organic solvents used to produce these NPs are toxic and can have destructive environmental impacts. Accordingly, there is a strong tendency to use reliable methods to synthesize NPs (2).
Nanobiotechnology was applied to biological systems (such as bacteria, fungi, and plants) to produce NPs. Presently, Fe2o3 NPs are produced using the “green synthesis method.” This method used biological sources, including bacteria, green algae, fungi, actinomycetes, and plant extracts (3). Green synthesis is clean, simple, eco-friendly, and non-toxic. On the other hand, the pathogenic effect of some organisms has been reported (4).
This study used Lactobacillus casei (PTCC: 1608). It is a rod, gram-positive organism known as a probiotic bacterium. Probiotics are defined as live microorganisms that, when consumed in sufficient quantities, provide a health benefit to the host (5).
Then, the cytotoxic effects of the synthesized Fe2o3 NPs were investigated on MCF-7 and human embryonic kidney 293 (HEK-293) cells as cancerous and normal cells, respectively.
Methods
Lactobacillus casei with (PTCC: 1608) identification was obtained from the microbial bank of the Iranian Research Organization for Science and Industry. The cytoplasmic extract was designed by freeze-thaw method and added to a 3-10M aqueous solution of Fe2o3 )Feo). Following regulating the pH of the solution to 5.6, it was incubated in darkness for 3 weeks at 37 °C (6).
The coprecipitation method was applied to synthesize the Fe3O4 NPs. Briefly, FeSO4 and NaOH were poured into separate beakers, 100 mL of deionized water was added to both beakers, and 2 capsule-rotating magnets were thrown inside. Then, both beakers were placed on a heater equipped with a magnetic stirrer to completely dissolve the materials. In the next step, FeCl3·6H2O was added to the solution. After creating an orange color solution, a black precipitate with a nanostructure was formed by adding NaOH. Finally, the excess salts were washed, and the precipitate was dried (7).
Eventually, a desiccator pulverized the response solution, and an X-ray diffraction (XRD) device (Philips PW 1710, NY, USA) was used to investigate the production of NPs. The sample was put on a glass substrate, and the scan was conducted with angle θ2 and in the range of 20 to 80 degrees. The voltage used was 40 kV, and the current was 30 mA. The particle size was calculated by the Debye-Scherrer equation handling with the following formula: D=0.9/ ß cosØA. A transmission electron microscope (Philips 208S 100 kV, Netherlands) was used to investigate the shape and size of NPs (6).
MCF-7 breast cancer cells and normal HEK-293 cells were acquired from the Pasteur Institute of Iran. For cell culture, cell lines were incubated in RPMI-1640 culture medium (Idea Zist Company, Iran) containing 10% FBS and 1% penicillin and streptomycin (Gibco, USA) in the presence of 5% CO2 at 37 °C. The cell environment was exchanged once every 3 days continuously; the cells reached a concentration of 80% for treatment. The cytotoxic effects of Fe2o3 NPs synthesized by the cytoplasmic extract of L. casei on MCF-7 breast cancer cells and normal HEK-293 cells were investigated using the MTT method (6,8). This process is based on the activity of mitochondrial dehydrogenases, which are significant markers in survival. Thus, the cells were cultured at a density of 104 cells in each well of a 96-well plate. Following 24 hours, the cell surface environment was modified and treated with varying concentrations of 10, 100, and 1000 μg/mL of Fe2o3 NPs for 48 hours, and cytotoxicity was evaluated by the MTT method.
The cytotoxic effects of Fe3O4 NPs synthesized on MCF-7 cancer cells and HEK-293 cells were studied using the Microculture Tetrazolium Test (MTT). MTT is reduced in the mitochondria of metabolically active cells by succinate dehydrogenase to yield a water-insoluble purple formazan crystal. To this purpose, 104 cells were cultured per well in a 96-well plate. After 24 hours of incubation, cell supernatants were removed, and they were treated with different concentrations of 10, 100, and 1000 µg/mL of Fe3O4 NPs for another 48 hours. Then, the cells were washed twice with PBS and incubated with 0.5 mg/mL MTT in a culture medium for 2.5 hours in a humidified atmosphere containing 5% CO2 at 37 °C. Following incubation, the MTT solution was removed, and formazan was extracted from cells with 200 μL of DMSO. The formazan-specific light absorption was then measured at 595 nm with a spectrophotometer (Rayleigh UV-2601, China). The experiment was repeated three times (9).
The data for each group were expressed as mean ± SD. An independent t test was used to compare viability between the 2 cell lines, and a 1-way analysis of variance (ANOVA) was used to analyze the results of the cytotoxic effect of the NPs on the cell lines. All analyses were performed using SPSS version 16 (SPSS Inc, Chicago, IL, USA). The statistical significance of all data was set at P < 0.05, P < 0.01, and P < 0.001.
Results
According to the XRD analysis results, the refractive peaks corresponding to angles θ2 or equal θ = 74°, 47°, 43° and 35°which can be indexed to (440), (400), (311), and 220. These peaks corresponded to the XRD pattern of Fe2o3. The NP size was calculated by the Debay-Scherrer relationship in the green synthesis of 15 nm and the chemical synthesis of 20 nm (Figures 1A and 1B).

The images show that the electron microscopy of Fe2o3 NPs synthesized by the green method has a spherical shape and a size of 15 nm in the chemical synthesis of 20 nm, which is consistent with the size obtained using the XRD diagram (Figures 2A and 2B).

Comparing the toxicity effects of green and chemically synthesized Fe2o3 NPs on MCF-7 cell lines
As shown in Figures 3A and 3B, after treatment with the synthesized NPs by each method, the viability of the MCF-7 cell line decreased in a concentration-dependent manner. Based on matching the results of chemically synthesized NPs with the green method (Figure 3C), it was confirmed that there was no significant difference in viability between the 2 methods at a concentration of 10 μg/mL. However, at a concentration of 100 μg/mL, the ability of viability in green synthesis was significantly reduced (P ≤ 0.01) compared to chemical synthesis. This reduction was also noticed at a concentration of 1000 μg/mL, which is insignificant.
As shown in Figures 3A and 3B, after treatment with the synthesized NPs by each method, the viability of the MCF-7 cell line decreased in a concentration-dependent manner. Based on matching the results of chemically synthesized NPs with the green method (Figure 3C), it was confirmed that there was no significant difference in viability between the 2 methods at a concentration of 10 μg/mL. However, at a concentration of 100 μg/mL, the ability of viability in green synthesis was significantly reduced (P ≤ 0.01) compared to chemical synthesis. This reduction was also noticed at a concentration of 1000 μg/mL, which is insignificant.

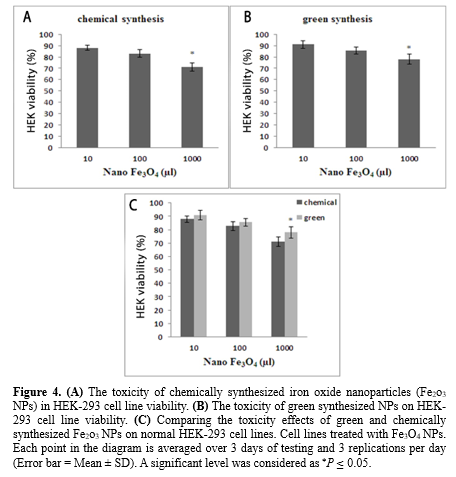
Comparing the toxicity effects of green and chemically synthesized Fe2o3 NPs on normal HEK-293 cell lines
According to Figures 4A and 4B, Fe2o3 NPs synthesized in both methods at a concentration of 1000 μL reduced the viability of the HEK-293 cell line by 15%. Based on matching the results of chemically and green synthesized NPs (Figure 4C), it is proven that there is no significant difference in viability between the 2 methods at concentrations of 10 and 100 μg/mL. However, at a concentration of 1000 μg/mL, the ability of viability in green synthesis increased significantly (P ≤ 0.05) compared to chemical synthesis.
According to Figures 4A and 4B, Fe2o3 NPs synthesized in both methods at a concentration of 1000 μL reduced the viability of the HEK-293 cell line by 15%. Based on matching the results of chemically and green synthesized NPs (Figure 4C), it is proven that there is no significant difference in viability between the 2 methods at concentrations of 10 and 100 μg/mL. However, at a concentration of 1000 μg/mL, the ability of viability in green synthesis increased significantly (P ≤ 0.05) compared to chemical synthesis.

Discussion
Cancer is the leading reason of mortality in developed countries and the second leading cause of death in developing countries. Despite many efforts in the prevention and treatment of cancer, the disease has grown exponentially. It is also considered a global killer. Despite the common treatments for cancer, one of the ways to improve the effects of chemotherapy drugs is to use compounds that are among the natural products that can reduce the side effects and have a strong effect on cancer cells. Reducing drug doses and providing greater efficiency in tumor diagnosis, tumor targeting and treatment, biocompatibility, and biodegradability are among the factors that make Fe2o3 NPs a suitable candidate for clinical applications, such as cell therapy, tissue repair, and drug guidance (10).
Today, the production of biological NPs is increasing due to their efficiency in medicine and life sciences. On the other hand, increasing awareness of green chemistry and biological processes has necessitated using environmentally friendly methods for the non-toxic production of biological nanomaterials. Although various biological pathways for the biological production of metal NPs are known, using plants or other intermediates to produce metal NPs is expensive and limited. Therefore, easy bio-preparation of NPs with size and shape control is of great importance in the proposed methods. In the meantime, using a microorganism that can stably produce NPs outside the cell, with a suitable particle size distribution, is an environmentally safe and interesting method due to its lower cost (11,12). Numerous studies have investigated the biosynthesis of NPs. Ghandehari et al showed the great potential of L. fermentum in the production of Fe2o3 NPs with a size of 20 nm (6). Comparing the results of this study with other studies showed that the chemical characteristics of Fe2o3 NPs taken from the cytoplasmic extract of L. casei are consistent with other studies. Previous studies have shown that microorganisms are able to synthesize metal NPs in the size of 1 to 200 nm using inexpensive and renewable reducing agents (13-15). The cytoplasmic extract of this bacterium seems to contain lactate or acetate-reducing agents, decreasing metal iron to Fe2o3 NPs. Many studies have shown that proteins and enzymes in the cytoplasmic extract of microorganisms play an important role in the synthesis of NPs (16, 17).
Comparing the cytotoxic effects of green and chemically synthesized Fe2o3 NPs showed that the synthesized green and chemical NPs with increasing concentrations revealed a great lethal impact on cancer cells, and synthesized green NPs at higher concentrations with a significant difference compared to chemical NPs reduced the survival of cancer cells. Nevertheless, the cytotoxic effects of NPs on normal cells also increased by increasing the concentration of NPs. The synthesized NPs manifested less cytotoxic effects on normal cells at a 100-μg/mL concentration; thus, it is suggested that this concentration can be used to inhibit the growth of cancer cells. Additional investigations have explained that the cytotoxic effects of NPs are concentration-dependent, bioparticles give more inhibitory effects on cancer cells compared to chemical NPs, and there is a direct relationship between NP concentration and NP toxicity on the cell lines tested. These results are perfectly consistent with this research (18-20). DNA damage is the most important cause of cancer cells, and this damage to genes can make a big difference between cancer cells and normal cells. These differences can also be used to recognize and destroy cancer cells accurately.
The mechanism of NP toxicity is not yet well understood; however, studies suggest that NPs play a key role in DNA damage, membrane destruction, and, ultimately, cell death by creating oxidative stress and lipid peroxidation, which can lead to effective therapies (21). Also, due to the high activity of mitochondria in the process of respiration of cancer cells compared to normal cells, a suitable substrate is provided for NPs to destroy cancer cells. Differences in the shape, size, and surface charge of NPs are other factors that contribute to differences in the toxicity of NPs between cancer cells and normal cells (22(. Therefore, Fe2o3 NPs synthesized by green and chemical methods can be used as a suitable solution in cancer treatment. Since green synthesis is an environmentally friendly method, it is recommended to use biological compounds to synthesize NPs to fight cancer.
Conclusion
Synthesizing Fe2o3 NPs by the cytoplasmic extract of L. casei was successful and had favorable cytotoxic effects on MCF-7 cancer cells. The findings were more significant than chemically synthesized NPs. The synthesized green NPs presented the most insignificant toxic effect on normal HEK-293 cells at low concentrations compared to chemically synthesized NPs. It seems that green synthesis can be regarded in cytotoxic investigations.
Acknowledgement
We gratefully acknowledge the assistance of Islamic Azad University of Flavarjan and the Department of Microbiology for their generous support, providing us with the necessary laboratories and equipment to conduct this study.
Funding sources
N/A.
Ethical statement
This study was approved by the Ethics Committee of Islamic Azad University, Falavarjan Branch, Isfahan, Iran.
Conflicts of interest
There is no conflict of interest.
Author contributions
This article was extracted from a master's thesis project in microbiology conducted by Dr Ghandhari, Dr Fatemi, Salehi, and Torabian at Islamic Azad University, Flavarjan Branch, Flavarjan, Isfahan, Iran.
Research Article: Research Article |
Subject:
Others
Received: 2021/07/25 | Accepted: 2021/10/13 | Published: 2024/02/26 | ePublished: 2024/02/26
Received: 2021/07/25 | Accepted: 2021/10/13 | Published: 2024/02/26 | ePublished: 2024/02/26
References
1. Zhang D, Ma X, Gu Y, Huang H, Zhang G. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front Chem.2020;8:1-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Koul B, Poonia AK, Yadav D, Jin JO. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules. 2021;11(6):886. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Vijayanandan AS, Balakrishnan RM. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J Environ Manage. 218;218:442-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Kim DY, Saratale RG, Shinde S, Syed A, Ameen F, Ghodake G. Green synthesis of silver nanoparticles using Laminaria japonica extract: Characterization and seedling growth assessment. J Clean Prod. 2018;172:2910-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Omidi B, Hashemi SJ, Bayat M, Larijani K. Biosynthesis of Silver Nanoparticles by Lactobacillus fermentum. Bull Environ Pharmacol Life Sci. 2014;3(12):186‐92. [View at Publisher] [Google Scholar]
6. Torabian P, Ghandehari F, Fatemi M. Evaluating Antibacterial Effect of Green Synthesis Oxide Iron Nanoparticles Using Cytoplasmic Extract of Lactobacillus casei. J Babol Univ Med Sci. 2019;21(1):237-41. [View at Publisher] [DOI] [Google Scholar]
7. Kandpal ND, Sah N, Loshali R, Joshi R, Prasad J. Co-precipitation method of synthesis and characterization of iron oxide nanoparticles. Journal of Scientific and Industrial Research. 2014;73(02):87-90. [View at Publisher] [Google Scholar]
8. Ghandehari F, Rezaee M, Fatemi M. Study on the antimicrobial effects of iron oxide nanoparticles synthesized by cytoplasmic extract of lactobacillus fermentum. New Cellular and Molecular Biotechnology Journal. 2021;9(36):89-96. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Rezaee M, Ghandehari F, Fatemi M, Fani M, Salehi D. Study on the Cytotoxic Effects of Iron Oxide Nanoparticles Synthesized by Cytoplasmic Extract of Lactobacillus Fermentum. Journal of Kerman University of Medical Sciences. 2022;29(1):31-8. [View at Publisher] [DOI] [Google Scholar]
10. Aghebati‐Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, et al. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. Journal of Cleaner Production. 2020;272(5):122880. [View at Publisher] [DOI] [Google Scholar]
12. Mandava K, Kadimcharla K, Keesara NR, Fatima SN, Bommena P, Batchu UR. Green Synthesis of Stable Copper Nanoparticles and Synergistic Activity with Antibiotics. Indian Journal of Pharmaceutical Sciences. 2017;79(5):695-700. [View at Publisher] [DOI] [Google Scholar]
13. Qamar H, Rehman S, Chauhan DK, Tiwari AK, Upmanyu V. Green Synthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanomaterial Derived from Momordica charantia. Int J Nanomedicine. 2020;15:2541-53. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Sayadi MH, Salmani N, Heidari A, Rezaei MR. Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surfaces and Interfaces. 2018;10:136-43. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Mirjani R, Faramarzi MA, Sharifzadeh M, Setayesh N, Khoshayand MR, Shahverdi AR. Biosynthesis of tellurium nanoparticles by Lactobacillus plantarum and the effect of nanoparticle‐enriched probiotics on the lipid profiles of mice. IET Nanobiotechnol. 2015;9(5):300-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Waghmare SR, Mulla MN, Marathe SR, Sonawane KD. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech. 2015;5(1):33-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Roychoudhury P, Gopal PK, Paul S, Pal R. Cyanobacteria assisted biosynthesis of silver nanoparticles-a potential antileukemic agent. Journal of Applied Phycology. 2016;28(6):3387-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Sayed Ahmad M, Mohamed Yasser M, Nageh Sholkamy E, Mohamed Ali A, Mohamed Mehanni M. Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int J Nanomedicine. 2015;10:3389-401. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Ramya S, Shanmugasundaram T, Balagurunathan R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol. 2015;32:30-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Abd-Elnaby HM, Abo-Elala GM, Abdel-Raouf UM, Hamed MM. Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. The Egyptian Journal of Aquatic Research. 2016;42(3):301-12. [View at Publisher] [DOI] [Google Scholar]
21. Fariq A, Khan T, Yasmin A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. Journal of Applied Biomedicine. 2017;15(4):241-8. [View at Publisher] [DOI] [Google Scholar]
22. Salem SS, Fouda A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: an Overview. Biol Trace Elem Res. 2021;199(1):344-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








