Volume 15, Issue 4 (Jul-Aug 2021)
mljgoums 2021, 15(4): 39-44 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khodamoradi S, Shahhosseiny M H, Mohammadian T, Ferdousi A. Evaluation of Role of Herpes Simplex Virus Types 1 and 2 and Cytomegalovirus in Alzheimer's Disease. mljgoums 2021; 15 (4) :39-44
URL: http://mlj.goums.ac.ir/article-1-1347-en.html
URL: http://mlj.goums.ac.ir/article-1-1347-en.html
1- Department Of Microbiology, Shahr-e-Qods Branch- Islamic Azad University, Tehran, Iran
2- Department Of Microbiology, Shahr-e-Qods Branch- Islamic Azad University, Tehran, Iran , shahhosseiny@qodsiau.ac.ir
2- Department Of Microbiology, Shahr-e-Qods Branch- Islamic Azad University, Tehran, Iran , shahhosseiny@qodsiau.ac.ir
Full-Text [PDF 706 kb]
(470 Downloads)
| Abstract (HTML) (1692 Views)
HSV1 and HSV2 were grown in a Vero cell line (Pasteur Institute of Iran) kept in RMPI medium. CMV was extracted from serum with a specific DNA titer. The PCR reaction was performed using PCR Master Mix, primers and extracted DNA samples. The results were analyzed on 2% agarose gel stained with SYBR green dye (SinaClon, Iran).
Statistical analysis was performed using SPSS 16.0 software. The results were expressed as mean ± standard deviations (SD). Data were analysed using one-way analysis of variance (ANOVA) followed by Dunnett’s new multiple range test at significance of 0.05.
RESULTS
Demographic profile and medical records of patients with AD are presented in table 1.
Table 1. Demographic date and medical records of the subjects
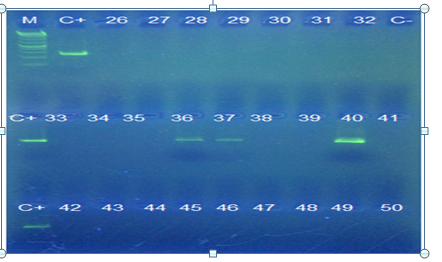
Figure 1. Gel electrophoresis of PCR products for detection of HSV-1 in samples collected from AD patients. M: 1kb DNA ladder (bioflux), C+: positive control, C-: negative control, lanes 51-75: negative samples.
.png)
Figure 2. Gel electrophoresis of PCR products for detection of HSV-2 in samples collected from AD patients. C+: positive control (231bp), C-: negative control, lanes 51,52,53,55-75: negative samples, lane 54: positive samples.

Figure 3. Gel electrophoresis of PCR products for detection of HSV-2 in samples collected from AD patients. M: 1kb DNA ladder (bioflux), C+: positive control (257 bp), C-: negative control, lanes 51-54,56-58,61,63-75: negative samples, lanes 55, 59, 60, 62: positive samples
Full-Text: (993 Views)
INTRODUCTION
Since the discovery of Alzheimer’s disease (AD) over 100 years ago, many studies have reported the pathological signs of this devastating disease (1,2). Development of AD is thought to be related to extracellular deposition of amyloid beta (Aβ) and intracellular hyperphosphorylated tau protein (3) that leads to synaptic dysfunction and decreased cognitive performance (4). It was believed that genetic and environmental factors may affect the onset of AD. Recently, it is hypothesized that AD may be linked to dysbiosis of microbes in the intestine. The hypothesis suggests that the intestinal flora is capable of affecting brain activity (3). The prevalence of mild to severe dementia is 5% among individuals aged 65 years and above. It is expected that the number of people with AD will reach 74.7 and 131.5 million by 2030 and 2050, respectively (5). In some patients with AD herpes simplex virus type 1 (HSV-1) weaken the immune system (6). Further experimental data proposed that other viruses, like cytomegalovirus (CMV), may also be involved in the pathogenesis of AD (7).
HSV types 1 (HSV-1) and 2 (HSV-2) are DNA viruses that encode more than 84 different proteins (8). These genes permit virus entry into cells for expression of certain genes that stop cellular and immune responses against viral infection. Both HSV-1 and HSV-2 protect cells against apoptosis (9). Initiation of apoptosis pathways occurs in a cell-type specific manner (10, 11, 12).
CMV is common in older adults and associated with mortality and cardiovascular disease (13, 14). Similar to HSVs, CMV constitutes a state of chronic infection, and viral reproduction must be concealed from the host immune response (15, 16). Most CMV brain infections occur in immunocompromised patients, such as transplant recipients, HIV patients and congenital CMV cases (17). Considering the importance of AD and its diagnosis, the present study aimed to investigate the prevalence of common DNA viruses in serum samples of AD patients in Tehran, Iran.
MATERIALS AND METHODS
Plasma samples were taken from 47 women and 53 men with AD in hospitals in Tehran, Iran. The samples were collected in sterile tubes and transferred to the microbiology laboratory. The samples were kept at -80 °C until DNA extraction. Quantitative and qualitative assessments of the extracted DNA were performed by spectrophotometry and agarose gel electrophoresis, respectively. Three primer pairs specific for HSV-1, HSV-2 and CMV were used to detect the viruses (Table 1) (19).
Table 1. The primers used for the detection of HSVs and CMV by PCR
Since the discovery of Alzheimer’s disease (AD) over 100 years ago, many studies have reported the pathological signs of this devastating disease (1,2). Development of AD is thought to be related to extracellular deposition of amyloid beta (Aβ) and intracellular hyperphosphorylated tau protein (3) that leads to synaptic dysfunction and decreased cognitive performance (4). It was believed that genetic and environmental factors may affect the onset of AD. Recently, it is hypothesized that AD may be linked to dysbiosis of microbes in the intestine. The hypothesis suggests that the intestinal flora is capable of affecting brain activity (3). The prevalence of mild to severe dementia is 5% among individuals aged 65 years and above. It is expected that the number of people with AD will reach 74.7 and 131.5 million by 2030 and 2050, respectively (5). In some patients with AD herpes simplex virus type 1 (HSV-1) weaken the immune system (6). Further experimental data proposed that other viruses, like cytomegalovirus (CMV), may also be involved in the pathogenesis of AD (7).
HSV types 1 (HSV-1) and 2 (HSV-2) are DNA viruses that encode more than 84 different proteins (8). These genes permit virus entry into cells for expression of certain genes that stop cellular and immune responses against viral infection. Both HSV-1 and HSV-2 protect cells against apoptosis (9). Initiation of apoptosis pathways occurs in a cell-type specific manner (10, 11, 12).
CMV is common in older adults and associated with mortality and cardiovascular disease (13, 14). Similar to HSVs, CMV constitutes a state of chronic infection, and viral reproduction must be concealed from the host immune response (15, 16). Most CMV brain infections occur in immunocompromised patients, such as transplant recipients, HIV patients and congenital CMV cases (17). Considering the importance of AD and its diagnosis, the present study aimed to investigate the prevalence of common DNA viruses in serum samples of AD patients in Tehran, Iran.
MATERIALS AND METHODS
Plasma samples were taken from 47 women and 53 men with AD in hospitals in Tehran, Iran. The samples were collected in sterile tubes and transferred to the microbiology laboratory. The samples were kept at -80 °C until DNA extraction. Quantitative and qualitative assessments of the extracted DNA were performed by spectrophotometry and agarose gel electrophoresis, respectively. Three primer pairs specific for HSV-1, HSV-2 and CMV were used to detect the viruses (Table 1) (19).
Table 1. The primers used for the detection of HSVs and CMV by PCR
| Virus | Primer Sequence | Product size for PCR (bp) | Target gene |
| HSV1 | H1F 5-TGGGACACATGCCTTCTTGG-3 H1R 5 CCCTTAGTCAGACTCTGTTACTTACCC-3 |
147 | Glycoprotein D |
| HSV2 | H2F 5-GTACAGACCTTCGGAGG-3 H2R 5-CGCTTCATCATGGGC-3 |
227 | Glycoprotein D |
| CMV | CMF 5-GTACACGCACGCTGGTTACC-3 CMR 5-GTAGAAAGCCTCGACATCGC-3 |
256 | IRL 11 |
HSV1 and HSV2 were grown in a Vero cell line (Pasteur Institute of Iran) kept in RMPI medium. CMV was extracted from serum with a specific DNA titer. The PCR reaction was performed using PCR Master Mix, primers and extracted DNA samples. The results were analyzed on 2% agarose gel stained with SYBR green dye (SinaClon, Iran).
Statistical analysis was performed using SPSS 16.0 software. The results were expressed as mean ± standard deviations (SD). Data were analysed using one-way analysis of variance (ANOVA) followed by Dunnett’s new multiple range test at significance of 0.05.
RESULTS
Demographic profile and medical records of patients with AD are presented in table 1.
Table 1. Demographic date and medical records of the subjects
| Male (n=53) | Blood types | A (n=7) | A+ (n=24) | Dysphoria (n=63) |
| AB-(n=5) | AB+ (n=11) | |||
| B+ (n=17) | O- (n=1) | |||
| O+ (n=35) | ||||
| Female (n=47) | History of infectious diseases (n=10) | Hallucination (n=33) | ||
| Married | Anemia (n=3) | Blood fats (n=38) | ||
| Mean age: 70.72±7 years | Fe deficiency (n=10) | Brain stroke (n=10) | ||
| Duration of disease: 10.34±7.23 months | P (normal), Ca (normal), Na (normal), K (normal) | Thyroid (n=15) | ||
| Insomnia (n=25) | Depression (n=44) | Heart attack (n=28) | ||
| Hypertension (n=37) | Diabetes (n=27) | CMV (n=27) | ||
| HSV1 (n=8) | ||||
| HSV2 (n=4) | ||||

Figure 1. Gel electrophoresis of PCR products for detection of HSV-1 in samples collected from AD patients. M: 1kb DNA ladder (bioflux), C+: positive control, C-: negative control, lanes 51-75: negative samples.
.png)
Figure 2. Gel electrophoresis of PCR products for detection of HSV-2 in samples collected from AD patients. C+: positive control (231bp), C-: negative control, lanes 51,52,53,55-75: negative samples, lane 54: positive samples.

DISCUSSION
This hypothesis that microorganisms might have an essential role in the development of AD was suggested by the Itzhaki’s group (3). It has been proposed that latent HSV-1 in the trigeminal ganglia could ascend along known nerve paths into the limbic system and regions of the brain most influenced in AD. HSV-1 seropositivity is associated with cognitive impairment in children and defective reading and visuospatial processing in middle-aged adults (22, 28).
CMV spreads from person to person via body fluids. Older adults have increased levels of IgG antibodies to CMV compared with younger individuals (20, 21, 22). Changes in cell-mediated immune parameters often happen with aging and can cause subclinical CMV reactivation (3).
Recent studies have provided further support for the recurrence of HSV-1 in brain in relation with the pathogenesis of AD (23). A review reported that these viruses can interact with other disease-modifying agents for initiation and/or progression of neurodegenerative disease (24). Apolipoprotein E is also involved in infection with HSV-1, hepatitis C and immune deficiency virus (24, 25). Bourgade et al. show that pathogens may be the most important contributor to the development of AD, especially in the case of HSV infection, that is often important at the same brain sites (26).
Itzhaki et al. confirmed the role of HSV-1 in AD in the last 8 years. Major advances in humans and mice have shown possibility of a hidden virus in the brain (20). Epidemiologic studies have shown the role of HSV-1 in AD (28, 29). Olsson et al. reported the prevalence of HSV to be 11.5% in human brain tumours. In addition, they showed that 86% of serum samples of healthy individuals were IgG positive for HSV.
Kristen et al. investigated HSV-2 infection with neurological symptoms such as AD in human neuroblastoma cells, and authors reported that HSV-2 infection leads to severe accumulation of phosphorylated peptides such as Aβ40 and Aβ42 amyloid peptides in human SK-N-MC neuroblastoma cells. A study in England showed that inflammation may be caused by a CNS infection or environmental infection (31). There are various microorganisms such as bacteria (mainly Troponium species), viruses (HSV-1) and yeasts (Candida species) in brain of subjects with AD.
Oral infection is also known as another possible cause of AD (31, 32). Viral agents of these infections occupy the central nervous system (32, 33). In another study, five different types of HSV were detected simultaneously using multiplex PCR in 86 patients with symptoms of meningitis and encephalitis. HSV-1 was detected in 3.5% of patients with meningoencephalitis, while HSV-2 was found in a new-born. In addition, varicella-zoster virus was detected in four patients with meningitis symptoms (4.6%) and in a new-born (19, 35).
CONCLUSION
CMV infection is associated with increased risk of AD and a quick rate of cognitive decline in elderly populations. Experimental data suggest participation of a polymicrobial community in the pathogenesis of AD.. Furthermore, neuronal infection with HSV-1, HSV-2 and CMV may help accumulate amyloid beta deposits and hyperphosphorylated tau that are related to AD pathology.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Dr. Ali Olfati for constructive criticism of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
CMV spreads from person to person via body fluids. Older adults have increased levels of IgG antibodies to CMV compared with younger individuals (20, 21, 22). Changes in cell-mediated immune parameters often happen with aging and can cause subclinical CMV reactivation (3).
Recent studies have provided further support for the recurrence of HSV-1 in brain in relation with the pathogenesis of AD (23). A review reported that these viruses can interact with other disease-modifying agents for initiation and/or progression of neurodegenerative disease (24). Apolipoprotein E is also involved in infection with HSV-1, hepatitis C and immune deficiency virus (24, 25). Bourgade et al. show that pathogens may be the most important contributor to the development of AD, especially in the case of HSV infection, that is often important at the same brain sites (26).
Itzhaki et al. confirmed the role of HSV-1 in AD in the last 8 years. Major advances in humans and mice have shown possibility of a hidden virus in the brain (20). Epidemiologic studies have shown the role of HSV-1 in AD (28, 29). Olsson et al. reported the prevalence of HSV to be 11.5% in human brain tumours. In addition, they showed that 86% of serum samples of healthy individuals were IgG positive for HSV.
Kristen et al. investigated HSV-2 infection with neurological symptoms such as AD in human neuroblastoma cells, and authors reported that HSV-2 infection leads to severe accumulation of phosphorylated peptides such as Aβ40 and Aβ42 amyloid peptides in human SK-N-MC neuroblastoma cells. A study in England showed that inflammation may be caused by a CNS infection or environmental infection (31). There are various microorganisms such as bacteria (mainly Troponium species), viruses (HSV-1) and yeasts (Candida species) in brain of subjects with AD.
Oral infection is also known as another possible cause of AD (31, 32). Viral agents of these infections occupy the central nervous system (32, 33). In another study, five different types of HSV were detected simultaneously using multiplex PCR in 86 patients with symptoms of meningitis and encephalitis. HSV-1 was detected in 3.5% of patients with meningoencephalitis, while HSV-2 was found in a new-born. In addition, varicella-zoster virus was detected in four patients with meningitis symptoms (4.6%) and in a new-born (19, 35).
CONCLUSION
CMV infection is associated with increased risk of AD and a quick rate of cognitive decline in elderly populations. Experimental data suggest participation of a polymicrobial community in the pathogenesis of AD.. Furthermore, neuronal infection with HSV-1, HSV-2 and CMV may help accumulate amyloid beta deposits and hyperphosphorylated tau that are related to AD pathology.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Dr. Ali Olfati for constructive criticism of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Research Article: Original Paper |
Subject:
Microbiology
Received: 2020/11/30 | Accepted: 2021/02/7 | Published: 2021/06/30 | ePublished: 2021/06/30
Received: 2020/11/30 | Accepted: 2021/02/7 | Published: 2021/06/30 | ePublished: 2021/06/30
References
1. Alzheimer A. Über eine eigenartige erkrankung der hirnrinde. Allg Z Psychiatr. Psychisch Gerichtl. Medcine. 1970; 64: 146-148. [View at Publisher] [Google Scholar]
2. Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clinical Anatomy. 1995; 8: 429-431. [DOI:10.1002/ca.980080612] [PubMed] [Google Scholar]
3. Esiri MM. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci. 1982 May;54(2):209-26. [View at Publisher] [DOI:10.1016/0022-510X(82)90183-6] [PubMed] [Google Scholar]
4. Sohrab SS, Suhail M, Ali A, Kamal MA, Husen A, Ahmad F, Azhar EI, Greig NH. Role of viruses, prions and miRNA in neurodegenerative disorders and dementia. Virusdisease. 2018; 29(4): 419-433. [DOI:10.1007/s13337-018-0492-y] [PubMed] [Google Scholar]
5. Fulop T, Witkowski JM, Bourgade K, Khalil A, Zerif E, Larbi A, et al. Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer's Disease? Front Aging Neurosci. 2018; 10: 224. [DOI:10.3389/fnagi.2018.00224] [PubMed] [Google Scholar]
6. Ball MJ. Limbic predilection in Alzheimer dementia: Is reactivated herpes virus involved? Canadian Journal of Neurology Science. 1982; 9: 303-306. [DOI:10.1017/S0317167100044115] [PubMed] [Google Scholar]
7. Kristen H, Santana S, Sastre I, Recuero M, Bullido MJ, Aldudo J. Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiology Aging. 2015; 36(10): 2737-47. [DOI:10.1016/j.neurobiolaging.2015.06.014] [PubMed] [Google Scholar]
8. Dupuis M, Hull R, Wang H, Nattanmai S. Molecular detection of viral causes of encephalitis and meningitis in New York State. Journal of Medicine Virology. 2011; 12(83): 2172-81. [View at Publisher] [DOI:10.1002/jmv.22169] [PubMed] [Google Scholar]
9. Hanger DP, Lau DH, Phillips EC, Bondulich MK, Guo T, Woodward BW. Intracellular and extracellular roles for tau in neurodegenerative disease. Journal of Alzheimer's Disease. 2014; 40(Suppl. 1): S37-S45. [DOI:10.3233/JAD-132054] [PubMed] [Google Scholar]
10. Olsson J, Lovheim H, Honkala E, Karhunen PJ, Elgh F, Kok EH. HSV presence in brains of individuals without dementia: the TASTY brain series. Disease Models & Mechanisms. 2016; 9(11): 1349-55. [View at Publisher] [DOI:10.1242/dmm.026674] [PubMed] [Google Scholar]
11. Steel AJ, Eslick GD. Herpes Viruses Increase the Risk of Alzheimer's Disease: A Meta-Analysis. J Alzheimers Dis. 2015;47(2):351-64. [DOI:10.3233/JAD-140822] [PubMed] [Google Scholar]
12. Lovheim H, Olsson J, Weidung B, Johansson A. Interaction between Cytomegalovirus and Herpes Simplex Virus Type 1 Associated with the risk of Alzheimer's Disease Development. Journal of Alzheimer's Disease. 2017; 61(3): 1. [DOI:10.3233/JAD-161305] [PubMed] [Google Scholar]
13. Perkins D. Targeting apoptosis in neurological disease using the herpes simplex virus. Journal of Cellular and Molecular Medicine. 2002; 6(3): 341-356. [DOI:10.1111/j.1582-4934.2002.tb00513.x] [PubMed] [Google Scholar]
14. Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathology. 2006; 111: 529-538. [View at Publisher] [DOI:10.1007/s00401-006-0037-0] [PubMed] [Google Scholar]
15. Barnes L, Capuano A, Aiello A, Turner A, Yolken R, Torrey EF, Bennett D. Cytomegalovirus Infection and Risk of Alzheimer Disease in Older Black and white Individuals. Journal of Infection Disease. 2015; 211: 230. [DOI:10.1093/infdis/jiu437] [PubMed] [Google Scholar]
16. Tudorache IF, Trusca VG, Gafencu AV. Apolipoprotein E-A Multifunctional Protein with Implications inVarious Pathologies as a Result of Its Structural Features. Computational and Structural Biotechnology Journal. 2017; 15: 359-65. [DOI:10.1016/j.csbj.2017.05.003] [PubMed] [Google Scholar]
17. Tarter KD, Simanek AM, Dowd JB, Aiello AE. Persistent viral pathogens and cognitive impairment across the life course in the third national health and nutrition examination survey. Journal of Infection Disease. 2014; 209: 837-844. [DOI:10.1093/infdis/jit616] [PubMed] [Google Scholar]
18. Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011; 2: 6. [DOI:10.1186/2042-4280-2-6] [PubMed] [Google Scholar]
19. Licastro F, Porcellini E. Persistent infections, immune-senescence and Alzheimer's disease. Oncoscience. 2016; 3(5-6): 135-42. [DOI:10.18632/oncoscience.309] [PubMed] [Google Scholar]
20. Itzhaki RF. Herpes simplex virus type 1 and Alzheimer's disease: possible mechanisms and signposts. The FASEB Journal. 2018; 31: 3216-26. [DOI:10.1096/fj.201700360] [PubMed] [Google Scholar]
21. Markoulatos P, Georgopoulou A. Laboratory Diagnosis of Common Herpesvirus Infections of the Central Nervous System by a Multiplex PCR Assay. Journal of Clinical Microbiology. 2001; 39(12): 4426-32. [DOI:10.1128/JCM.39.12.4426-4432.2001] [PubMed] [Google Scholar]
22. Terry RD, Masliah E, Salmon DP, Butters N, and DeTeresa R, Hill R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals Neurology. 1991; 30: 572-580. [DOI:10.1002/ana.410300410] [PubMed] [Google Scholar]
23. Itzhaki RF. Herpes and Alzheimer's disease: Subversion in the Central Nervous System and How It Might Be Halted. Journal of Alzheimer's Disease. 2016; 54: 13-3721. [DOI:10.3233/JAD-160607] [PubMed] [Google Scholar]
24. Gholamzadeh S, Heshmati B, Mani A, Petramfar P, Baghery Z. The prevalence of Alzheimer's disease; its risk and protective factors among the elderly population in Iran. Shiraz E-Medical Journal. 2017; 18(9): e57576. [View at Publisher] [DOI:10.5812/semj.57576] [Google Scholar]
25. Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991; 72 ( Pt 9): 2059-64. [DOI:10.1099/0022-1317-72-9-2059] [PubMed] [Google Scholar]
26. Baringer JR, Pisani P. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Annals of Neurology. 1994; 36: 823-829. [View at Publisher] [DOI:10.1002/ana.410360605] [PubMed] [Google Scholar]
27. Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, Schneider JA. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013; 208(4): 564-72. [DOI:10.1093/infdis/jit210] [PubMed] [Google Scholar]
28. Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer's disease? Journal of Oral Microbiology. 2015; 7: 29143. [DOI:10.3402/jom.v7.29143] [PubMed] [Google Scholar]
29. Hogestyn JM, Mock DJ, Mayer-Proschel M. Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regeneration Research. 2018; 13(2): 211-21. [DOI:10.4103/1673-5374.226380] [PubMed] [Google Scholar]
30. woods AG. HSV Linked to Alzheimer Disease. Neurology Live. 2014. [View at Publisher]
31. Mancuso L, Cao G. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicology Mechanisms and Methods. 2014; 24: 449-454. [DOI:10.3109/15376516.2014.928920] [PubMed] [Google Scholar]
32. Bourgade K, Dupuis G, Frost EH, Fulop T. Anti-Viral Properties of Amyloid-beta Peptides. Journal of Alzheimer's Disease. 2016; 54(3): 859-78. [DOI:10.3233/JAD-160517] [PubMed] [Google Scholar]
33. Tabarraei H, Hassanb J, Parvizia MR, Golshahic H, Keshavarz-Tarikhi H. Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicology Reports 2019; 6: 869-874. [View at Publisher] [DOI:10.1016/j.toxrep.2019.08.010] [PubMed] [Google Scholar]
34. Tabarraei H, Hassanb J, Sadat Mosavi S. Determination of LD50 of some essential oils and histopathological changes in short-term exposure to one of them in rainbow trout (Oncorhynchus mykiss). Toxicology Research and Application. 2019; 3:1-7. [View at Publisher] [DOI:10.1177/2397847318820719] [Google Scholar]
35. Emami S, Olfati A. Effects of dietary supplementing of Spirulina platensis and Chlorella vulgaris microalgae on hematologic parameters in streptozotocin-induced diabetic rats. Iranian Journal of Pediatric Hematology and Oncology 2017; 7(3):163-170. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









