Volume 17, Issue 2 (Mar-Apr 2023)
mljgoums 2023, 17(2): 14-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zakeri M, alimoradi E, Seyyedhashemi E, Marhamati S, Tajari V, Joshaghani H. Evaluation of Anti-Nuclear Antibodies and Anti-dsDNA Serum Levels in Patients with Suspected Systemic Lupus Erythematosus. mljgoums 2023; 17 (2) :14-19
URL: http://mlj.goums.ac.ir/article-1-1329-en.html
URL: http://mlj.goums.ac.ir/article-1-1329-en.html
Mana Zakeri1 

 , Elham Alimoradi2
, Elham Alimoradi2 

 , Effat Seyyedhashemi3
, Effat Seyyedhashemi3 

 , Shayan Marhamati4
, Shayan Marhamati4 

 , Vahid Tajari5
, Vahid Tajari5 

 , Hamidreza Joshaghani
, Hamidreza Joshaghani 

 6
6


 , Elham Alimoradi2
, Elham Alimoradi2 

 , Effat Seyyedhashemi3
, Effat Seyyedhashemi3 

 , Shayan Marhamati4
, Shayan Marhamati4 

 , Vahid Tajari5
, Vahid Tajari5 

 , Hamidreza Joshaghani
, Hamidreza Joshaghani 

 6
6
1- Department of biology, Islamic Azad University, Tehran Medical Branch, Tehran, Iran
2- Department of biology ,faculty of science ,Razi university ,kermanshah ,iran
3- Department of Molecular Medicine, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
4- department of Biochemistry, golestan University of Medical Sciences, Gorgan, Iran
5- Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran
6- Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran
2- Department of biology ,faculty of science ,Razi university ,kermanshah ,iran
3- Department of Molecular Medicine, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
4- department of Biochemistry, golestan University of Medical Sciences, Gorgan, Iran
5- Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran
6- Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran
Keywords: Antibodies, Antinuclear [MeSH], Lupus Erythematosus, Systemic [MeSH], ELISA [MeSH], Diagnosis [MeSH]
Full-Text [PDF 512 kb]
(291 Downloads)
| Abstract (HTML) (1330 Views)
Full-Text: (349 Views)
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects many organs and tissues in the body, in which vital organs and tissues are invaded by pathogenic immune complexes and antibodies (1). Although the exact etiology of SLE is not completely elucidated, several studies have shown that factors such as viral infections, drug side effects, chronic depression, trauma, or environmental stress in addition to genetic background may predispose individuals to autoimmune diseases, such as SLE (2). Given the lack of definitive treatment for SLE, the patients experience stages of relapse or partial recovery for the rest of their life (3). The disease has a wide and diversified geographical distribution in different regions of the world. The prevalence of SLE in Iran is estimated at 40 per 100,000 people according to a large study by the Tehran Rheumatology Research Center (4). In Iran, 90% of the affected cases are women, which is almost equal to the global reported ratio (5).
As an autoimmune disease, SLE is caused by abnormal innate and adaptive immune responses. Regarding the disruption of adaptive immunity, overactive B lymphocytes give raise to autoantibodies against cellular components, leading to the activation of persistent inflammatory reactions and damage to vital organs, including kidneys, heart, lungs, and joints (6). Patients may express a variety of clinical symptoms including fever, skin lesions, hair loss, pain and fatigue, systemic inflammation, movement disorders, osteoporosis, and cognitive and mental disorders, depending on the site of involvement (7).
The American College of Rheumatology’s classification criteria is currently used as a global standard for diagnosing SLE (8). Among all accepted criteria, the presence of anti-nuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anti-phospholipid antibodies are known as reliable biomarkers in detecting SLE (9). Moreover, the Systemic Lupus International Collaborating Clinics has recently reported that the presence of elevated ANA and anti-ds DNA antibodies are of great importance and could be sufficient in diagnosing SLE (10). Furthermore, the anti-dsDNA antibody is one of the few antibodies which could be measured quantitatively and is considered a valuable marker for monitoring the SLE disease activity (11). Several studies have evaluated the clinical application of anti-dsDNA and ANA in the diagnosis and prognosis of SLE in different populations with controversial findings (12-15). To the best of our knowledge, no study has evaluated the serum levels of anti-dsDNA and ANA antibodies in rheumatology clinics of Gorgan (northeastern Iran) and their possible clinical applications. Accordingly, we aimed to investigate the serum levels of anti-dsDNA and ANA antibodies in the diagnosis of SLE and their relationship with disease activity and clinical/laboratory manifestations in suspected patients referring to rheumatology clinics in Gorgan, Iran.
MATERIALS AND METHODS
All individuals suspected of having SLE (N=668) who had been referred from rheumatology clinics to the Kavosh clinical laboratory in Gorgan from December 2019 to September 2020 were included in this cross-sectional study. The Ethics Committee of Golestan University of Medical Sciences approved the study protocols (ethical code approval: IR.GOUMS.REC.1399.388), and informed consent was obtained from all participants. All subjects who had been tested for ANA and anti-dsDNA levels and completed a disease checklist were included in the study. Pregnant individuals, cancer patients, and those who were unwilling to participate in the study were excluded.
Whole blood samples (5 ml) were taken from all suspected patients. Plasma was separated by centrifugation at 3,000 rpm for 5 minutes. The plasma samples were stored at -20 °C until use. All laboratory and clinical parameters were also recorded. The plasma levels of ANA and anti-dsDNA antibodies were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (LifeSpan BioSciences, USA). Moreover, the serum levels of C3, C4, and CH50 were also quantified using commercially available ELISA kits (LifeSpan BioSciences, USA).
Data analysis was carried out by using SPSS software (version 16). Independent samples t-test was used for intergroup comparison, while the relationship between quantitative variables was examined through correlation tests. A p-value less than 0.05 was considered statistically significant.
RESULTS
As shown in table 1, the chi-square test results showed that ANA-positive and ANA-negative individuals were not homogeneous in terms of gender distribution (p<0.01).
Table 1- Gender distribution in ANA-positive and ANA-negative individuals
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects many organs and tissues in the body, in which vital organs and tissues are invaded by pathogenic immune complexes and antibodies (1). Although the exact etiology of SLE is not completely elucidated, several studies have shown that factors such as viral infections, drug side effects, chronic depression, trauma, or environmental stress in addition to genetic background may predispose individuals to autoimmune diseases, such as SLE (2). Given the lack of definitive treatment for SLE, the patients experience stages of relapse or partial recovery for the rest of their life (3). The disease has a wide and diversified geographical distribution in different regions of the world. The prevalence of SLE in Iran is estimated at 40 per 100,000 people according to a large study by the Tehran Rheumatology Research Center (4). In Iran, 90% of the affected cases are women, which is almost equal to the global reported ratio (5).
As an autoimmune disease, SLE is caused by abnormal innate and adaptive immune responses. Regarding the disruption of adaptive immunity, overactive B lymphocytes give raise to autoantibodies against cellular components, leading to the activation of persistent inflammatory reactions and damage to vital organs, including kidneys, heart, lungs, and joints (6). Patients may express a variety of clinical symptoms including fever, skin lesions, hair loss, pain and fatigue, systemic inflammation, movement disorders, osteoporosis, and cognitive and mental disorders, depending on the site of involvement (7).
The American College of Rheumatology’s classification criteria is currently used as a global standard for diagnosing SLE (8). Among all accepted criteria, the presence of anti-nuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anti-phospholipid antibodies are known as reliable biomarkers in detecting SLE (9). Moreover, the Systemic Lupus International Collaborating Clinics has recently reported that the presence of elevated ANA and anti-ds DNA antibodies are of great importance and could be sufficient in diagnosing SLE (10). Furthermore, the anti-dsDNA antibody is one of the few antibodies which could be measured quantitatively and is considered a valuable marker for monitoring the SLE disease activity (11). Several studies have evaluated the clinical application of anti-dsDNA and ANA in the diagnosis and prognosis of SLE in different populations with controversial findings (12-15). To the best of our knowledge, no study has evaluated the serum levels of anti-dsDNA and ANA antibodies in rheumatology clinics of Gorgan (northeastern Iran) and their possible clinical applications. Accordingly, we aimed to investigate the serum levels of anti-dsDNA and ANA antibodies in the diagnosis of SLE and their relationship with disease activity and clinical/laboratory manifestations in suspected patients referring to rheumatology clinics in Gorgan, Iran.
MATERIALS AND METHODS
All individuals suspected of having SLE (N=668) who had been referred from rheumatology clinics to the Kavosh clinical laboratory in Gorgan from December 2019 to September 2020 were included in this cross-sectional study. The Ethics Committee of Golestan University of Medical Sciences approved the study protocols (ethical code approval: IR.GOUMS.REC.1399.388), and informed consent was obtained from all participants. All subjects who had been tested for ANA and anti-dsDNA levels and completed a disease checklist were included in the study. Pregnant individuals, cancer patients, and those who were unwilling to participate in the study were excluded.
Whole blood samples (5 ml) were taken from all suspected patients. Plasma was separated by centrifugation at 3,000 rpm for 5 minutes. The plasma samples were stored at -20 °C until use. All laboratory and clinical parameters were also recorded. The plasma levels of ANA and anti-dsDNA antibodies were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (LifeSpan BioSciences, USA). Moreover, the serum levels of C3, C4, and CH50 were also quantified using commercially available ELISA kits (LifeSpan BioSciences, USA).
Data analysis was carried out by using SPSS software (version 16). Independent samples t-test was used for intergroup comparison, while the relationship between quantitative variables was examined through correlation tests. A p-value less than 0.05 was considered statistically significant.
RESULTS
As shown in table 1, the chi-square test results showed that ANA-positive and ANA-negative individuals were not homogeneous in terms of gender distribution (p<0.01).
Table 1- Gender distribution in ANA-positive and ANA-negative individuals
| p-value | ANA group | |||
| Negative | Positive | |||
| <0.001 | 337 | 88 | Female | Gender |
| 59.6% | 85.4% | |||
| 228 | 15 | Male | ||
| 40.4% | 14.6% | |||
As shown in figure 1, the mean age of the subjects did not differ significantly between the two groups (p=0.776).

Figure 1- Comparison of the mean age of individuals between ANA-positive and ANA-negative subjects (based on the independent t-test)
.PNG)
Figure2-Comparison of the mean of ANA and anti-ds DNA levelsin ANA positive and negative SUBGROUOS

Figure 3- Comparison of the mean serum C3 levels between the ANA-positive and -negative groups
.PNG)
Figure 4- Comparison of the mean serum C4 levels between the ANA-positive and -negative groups

Figure 5- Comparison of the mean serum CH50 levels between the ANA-positive and -negative groups
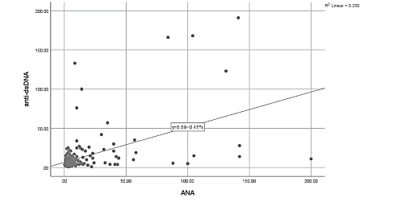
Figure 6- The correlation between ANA and anti-dsDNA levels based on the Pearson correlation coefficient
As demonstrated in figure 2, the mean of ANA was 36.44 U/mL in the ANA-positive group and 3.34 U/mL in the negative group (P -value < 0.001). We also evaluated and compared the mean anti-dsDNA values between ANA-positive and -negative groups using independent samples t-test and covariance analysis (adjusted by gender). The mean level of anti-dsDNA antibodies in ANA-positive individuals (30.15 IU/mL) was significantly higher than that in ANA-negative individuals (15.01 IU/mL) (p<0.001).
We compared the mean serum levels of C3, C4, and CH50 in the ANA-positive and -negative groups using the Mann–Whitney U test. Our findings showed that there was no significant difference between the two groups in terms of mean levels of C3 (p= 0.233), C4 (p=0.415), and CH50 (p =0.482) (Figures 3-5).
Based on the results of the Pearson correlation coefficient, there was a significant positive correlation between the mean levels of ANA and anti-dsDNA antibodies (p<0.001, r=0.50) (Figure 6).
DISCUSSION
Systemic lupus erythematosus is an autoimmune disease, caused by abnormal innate and adaptive immune responses (16). Anti-nuclear antibodies are a special group of antibodies with the ability to bind to and destroy specific structures in the cell nucleus (17). Anti-dsDNA antibodies are also a heterogeneous group of ANAs that leads to autoimmune disorders. Elevated levels of these antibodies have been linked with SLE (18). In the present study, we assessed the levels of anti-dsDNA and ANA antibodies in patients with suspected SLE. Based on the findings, 15.42% of suspected individuals who had been referred to the rheumatology clinics in Gorgan, northeastern Iran were positive for ANA and anti-dsDNA tests, indicating that these participants might have developed SLE (8). We also found a strong positive correlation between ANA and anti-dsDNA levels, delineating the simultaneous diagnostic value of these two markers in SLE. In this regard, Bardin et al. (2009) showed that the diagnostic sensitivity of SLE may increase from 68-70% to 78% when ANA and anti-dsDNA are measured simultaneously (19). Infantino et al. (2018) reported that ANA antibodies are more sensitive and specific biomarkers for the diagnosis of SLE than anti-dsDNA antibodies (20). However, they also recommended that the two antibodies should be combined for the diagnosis of SLE (20). In the present study, it was also shown that anti-dsDNA levels in ANA-positive subjects were significantly higher than in their ANA-negative counterparts, which is consistent with the findings of Infantino et al. (20). In the present study, the diagnostic values of these two markers for the diagnosis of SLE were not evaluated in combination and separately. However, in ANA-positive individuals, there might be a significant positive correlation between ANA and anti-dsDNA levels. Given the fact that ANA and anti-dsDNA levels could be associated with SLE disease activity, the detection of these antibodies might be beneficial for the estimation of prognosis and treatment efficiency, especially in neglected cases (21).
In the present study, although ANA was reported to be positive in 15.42% of cases with suspected SLE, no comprehensive assessment of the prevalence of SLE was performed, which is a limitation of our study. Based on ANA positivity, 85.4% of positive SLE patients in our study were female, which is lower than the rates reported in a previous study in Iran (22). Iran has a population of different ethnicities and diversified regional subgroups. Therefore, the results of the current study could provide useful information about the impact of genetic and environmental factors on the diagnosis of SLE.
CONCLUSION
Our findings showed that more than 85% of individuals with suspected SLE were positive for ANAand the majority of suspects were female. Based on the results, anti-dsDNA levels are significantly higher in ANA-positive individuals than in ANA-negative counterparts. There is also a positive correlation between ANA and anti-dsDNA levels, especially in the ANA-positive group. It is recommended that in subsequent studies, all individuals would be followed up clinically for at least 6 months after the test, and the diagnostic and prognostic value of ANA and anti-dsDNA antibodies would be calculated.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
FUNDING
The current research was derived from a research project supported by the Department of Research and Technology of Golestan University of Medical Sciences.
Ethics approvals and consent to participate
The Ethics Committee of Golestan University of Medical Sciences approved the study protocols (ethical code approval: IR.GOUMS.REC.1399.388), and informed consent was obtained from all participants.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.

Figure 1- Comparison of the mean age of individuals between ANA-positive and ANA-negative subjects (based on the independent t-test)
.PNG)
Figure2-Comparison of the mean of ANA and anti-ds DNA levelsin ANA positive and negative SUBGROUOS

Figure 3- Comparison of the mean serum C3 levels between the ANA-positive and -negative groups
.PNG)
Figure 4- Comparison of the mean serum C4 levels between the ANA-positive and -negative groups

Figure 5- Comparison of the mean serum CH50 levels between the ANA-positive and -negative groups

Figure 6- The correlation between ANA and anti-dsDNA levels based on the Pearson correlation coefficient
As demonstrated in figure 2, the mean of ANA was 36.44 U/mL in the ANA-positive group and 3.34 U/mL in the negative group (P -value < 0.001). We also evaluated and compared the mean anti-dsDNA values between ANA-positive and -negative groups using independent samples t-test and covariance analysis (adjusted by gender). The mean level of anti-dsDNA antibodies in ANA-positive individuals (30.15 IU/mL) was significantly higher than that in ANA-negative individuals (15.01 IU/mL) (p<0.001).
We compared the mean serum levels of C3, C4, and CH50 in the ANA-positive and -negative groups using the Mann–Whitney U test. Our findings showed that there was no significant difference between the two groups in terms of mean levels of C3 (p= 0.233), C4 (p=0.415), and CH50 (p =0.482) (Figures 3-5).
Based on the results of the Pearson correlation coefficient, there was a significant positive correlation between the mean levels of ANA and anti-dsDNA antibodies (p<0.001, r=0.50) (Figure 6).
DISCUSSION
Systemic lupus erythematosus is an autoimmune disease, caused by abnormal innate and adaptive immune responses (16). Anti-nuclear antibodies are a special group of antibodies with the ability to bind to and destroy specific structures in the cell nucleus (17). Anti-dsDNA antibodies are also a heterogeneous group of ANAs that leads to autoimmune disorders. Elevated levels of these antibodies have been linked with SLE (18). In the present study, we assessed the levels of anti-dsDNA and ANA antibodies in patients with suspected SLE. Based on the findings, 15.42% of suspected individuals who had been referred to the rheumatology clinics in Gorgan, northeastern Iran were positive for ANA and anti-dsDNA tests, indicating that these participants might have developed SLE (8). We also found a strong positive correlation between ANA and anti-dsDNA levels, delineating the simultaneous diagnostic value of these two markers in SLE. In this regard, Bardin et al. (2009) showed that the diagnostic sensitivity of SLE may increase from 68-70% to 78% when ANA and anti-dsDNA are measured simultaneously (19). Infantino et al. (2018) reported that ANA antibodies are more sensitive and specific biomarkers for the diagnosis of SLE than anti-dsDNA antibodies (20). However, they also recommended that the two antibodies should be combined for the diagnosis of SLE (20). In the present study, it was also shown that anti-dsDNA levels in ANA-positive subjects were significantly higher than in their ANA-negative counterparts, which is consistent with the findings of Infantino et al. (20). In the present study, the diagnostic values of these two markers for the diagnosis of SLE were not evaluated in combination and separately. However, in ANA-positive individuals, there might be a significant positive correlation between ANA and anti-dsDNA levels. Given the fact that ANA and anti-dsDNA levels could be associated with SLE disease activity, the detection of these antibodies might be beneficial for the estimation of prognosis and treatment efficiency, especially in neglected cases (21).
In the present study, although ANA was reported to be positive in 15.42% of cases with suspected SLE, no comprehensive assessment of the prevalence of SLE was performed, which is a limitation of our study. Based on ANA positivity, 85.4% of positive SLE patients in our study were female, which is lower than the rates reported in a previous study in Iran (22). Iran has a population of different ethnicities and diversified regional subgroups. Therefore, the results of the current study could provide useful information about the impact of genetic and environmental factors on the diagnosis of SLE.
CONCLUSION
Our findings showed that more than 85% of individuals with suspected SLE were positive for ANAand the majority of suspects were female. Based on the results, anti-dsDNA levels are significantly higher in ANA-positive individuals than in ANA-negative counterparts. There is also a positive correlation between ANA and anti-dsDNA levels, especially in the ANA-positive group. It is recommended that in subsequent studies, all individuals would be followed up clinically for at least 6 months after the test, and the diagnostic and prognostic value of ANA and anti-dsDNA antibodies would be calculated.
ACKNOWLEDGEMENTS
None.
DECLARATIONS
FUNDING
The current research was derived from a research project supported by the Department of Research and Technology of Golestan University of Medical Sciences.
Ethics approvals and consent to participate
The Ethics Committee of Golestan University of Medical Sciences approved the study protocols (ethical code approval: IR.GOUMS.REC.1399.388), and informed consent was obtained from all participants.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Research Article: Original Paper |
Subject:
Microbiology
Received: 2020/09/16 | Accepted: 2021/06/16 | Published: 2023/03/14 | ePublished: 2023/03/14
Received: 2020/09/16 | Accepted: 2021/06/16 | Published: 2023/03/14 | ePublished: 2023/03/14
References
1. Mok C, Lau C. Pathogenesis of systemic lupus erythematosus. Journal of clinical pathology. 2003; 56(7): 481-90. [View at Publisher] [DOI:10.1136/jcp.56.7.481] [PubMed] [Google Scholar]
2. Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Practice & Research Clinical Rheumatology. 2018; 32(2): 188-205. [View at Publisher] [DOI:10.1016/j.berh.2018.09.004] [PubMed] [Google Scholar]
3. Kuhn A, Bonsmann G, Anders H-J, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Deutsches Ärzteblatt International. 2015;112(25):423. [DOI:10.3238/arztebl.2015.0423] [PubMed] [Google Scholar]
4. Jamshidi A-R, Tehrani-Banihashemi A, Dahaghin S, Gholami J, Froozanfar M-H, Akhlaghi M, et al. Clinical hand osteoarthritis in Tehran: prevalence, signs, symptoms, and pattern-COPCORD Stage I, Iran Study. The Journal of rheumatology. 2008;35(7):1467-9. [View at Publisher] [PubMed] [Google Scholar]
5. Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clinical Immunology. 2013;149(2):211-8. [View at Publisher] [DOI:10.1016/j.clim.2013.03.003] [PubMed] [Google Scholar]
6. Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. The Journal of experimental medicine. 2005; 201(5): 703-11. [View at Publisher] [DOI:10.1084/jem.20042251] [PubMed] [Google Scholar]
7. Smith PP, Gordon C. Systemic lupus erythematosus: clinical presentations. Autoimmunity reviews. 2010;10(1):43-5. [View at Publisher] [DOI:10.1016/j.autrev.2010.08.016] [PubMed] [Google Scholar]
8. Aringer M. EULAR/ACR classification criteria for SLE. Semin Arthritis Rheum. 2019; 49(3S): S14-S17. [DOI:10.1016/j.semarthrit.2019.09.009] [PubMed]
9. Gheita T, Abaza N, Hammam N, Mohamed A, El-Gazzar I, Eissa A. Anti-dsDNA titre in female systemic lupus erythematosus patients: relation to disease manifestations, damage and antiphospholipid antibodies. Lupus. 2018;27(7):1081-7. [View at Publisher] [DOI:10.1177/0961203318760209] [PubMed] [Google Scholar]
10. Ighe A, Dahlström Ö, Skogh T, Sjöwall C. Application of the 2012 Systemic Lupus International Collaborating Clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis research & therapy. 2015;17(1):1-8. [View at Publisher] [DOI:10.1186/s13075-015-0521-9] [PubMed] [Google Scholar]
11. Isenberg D, Manson J, Ehrenstein M, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey's end? 2007. [View at Publisher] [DOI:10.1093/rheumatology/kem112] [PubMed] [Google Scholar]
12. Fu SM, Dai C, Zhao Z, Gaskin F. Anti-dsDNA Antibodies are one of the many autoantibodies in systemic lupus erythematosus. F1000Research. 2015;4(F1000 Faculty Rev). [View at Publisher] [DOI:10.12688/f1000research.6875.1] [PubMed] [Google Scholar]
13. Bragazzi NL, Watad A, Damiani G, Adawi M, Amital H, Shoenfeld Y. Role of anti-DNA auto-antibodies as biomarkers of response to treatment in systemic lupus erythematosus patients: hypes and hopes. Insights and implications from a comprehensive review of the literature. Expert review of molecular diagnostics. 2019;19(11):969-78. [View at Publisher] [DOI:10.1080/14737159.2019.1665511] [PubMed] [Google Scholar]
14. Herbst R, Liu Z, Jallal B, Yao Y. Biomarkers for systemic lupus erythematosus. Int J Rheum Dis. 2012; 15(5): 433-44. [DOI:10.1111/j.1756-185X.2012.01764.x] [PubMed]
15. Gensous N, Marti A, Barnetche T, Blanco P, Lazaro E, Seneschal J, et al. Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis research & therapy. 2017;19(1):1-12. [View at Publisher] [DOI:10.1186/s13075-017-1442-6] [PubMed] [Google Scholar]
16. Mohammadi S, Sedighi S, Memarian A. IL-17 is aberrantly overexpressed among under-treatment systemic lupus erythematosus patients. Iranian journal of pathology. 2019;14(3):236. [View at Publisher] [DOI:10.30699/IJP.2019.94878.1934] [PubMed] [Google Scholar]
17. Pisetsky DS, Bossuyt X, Meroni PL. ANA as an entry criterion for the classification of SLE. Autoimmunity reviews. 2019;18(12):102400. [View at Publisher] [DOI:10.1016/j.autrev.2019.102400] [PubMed] [Google Scholar]
18. Granito A, Muratori L, Tovoli F, Muratori P. Diagnostic role of anti-dsDNA antibodies: do not forget autoimmune hepatitis. Nature Reviews Rheumatology. 2021;17(4):244-. [View at Publisher] [DOI:10.1038/s41584-021-00573-7] [PubMed] [Google Scholar]
19. Bardin N, Desplat-Jego S, Daniel L, Jourde Chiche N, Sanmarco M. BioPlex™ 2200 multiplexed system: Simultaneous detection of anti-dsDNA and anti-chromatin antibodies in patients with systemic lupus erythematosus. Autoimmunity. 2009; 42(1): 63-8. [View at Publisher] [DOI:10.1080/08916930802354906] [PubMed] [Google Scholar]
20. Infantino M, Grossi V, Benucci M, Li Gobbi F, Damiani A, Manfredi M. The impact of biological treatments on the anti-dsDNA and anti-nucleosome tests. Lupus. 2018;27(1):40-8. [View at Publisher] [DOI:10.1177/0961203317709344] [PubMed] [Google Scholar]
21. Almogren A. Anti-double stranded antibody. Association with titers and fluorescence patterns of anti-nuclear antibody in systemic lupus erythematosus. Saudi Med J. 2010;31(1):32-6. [PubMed] [Google Scholar]
22. Fatemi A, Matinfar M, Sayedbonakdar Z, Maracy M, Karimzadeh H, Saber M, et al. Outcome of adult onset systemic lupus erythematosus in Iran. Lupus. 2014; 23(11): 1211-6. [View at Publisher] [DOI:10.1177/0961203314534304] [PubMed] [Google Scholar]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




