Volume 16, Issue 3 (May-Jun 2022)
mljgoums 2022, 16(3): 1-6 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pandit A, Siddegowda M, Shoba K L. Evaluation of Soft Tissue Tumors by Fine Needle Aspiration Cytology and Its Correlation with Histopathology: A Retrospective Study in a Tertiary Care Center. mljgoums 2022; 16 (3) :1-6
URL: http://mlj.goums.ac.ir/article-1-1468-en.html
URL: http://mlj.goums.ac.ir/article-1-1468-en.html
1- Department of Pathology, Mandya Institute of Medical Sciences, Mandya, India , p_apoorva@ymail.com
2- Department of Pathology, Mandya Institute of Medical Sciences, Mandya, India
2- Department of Pathology, Mandya Institute of Medical Sciences, Mandya, India
Full-Text [PDF 913 kb]
(467 Downloads)
| Abstract (HTML) (1342 Views)
Soft tissues are non-epithelial extraskeletal tissues of the body. They comprise of skeletal muscles, fat, blood vessels, nerves, and supportive connective tissue. Soft tissues are derived from the mesoderm and their main function is to provide support to various organs. Soft tissue tumors (STTs) are a heterogeneous group of tumors that are classified according to their differentiation into the adult tissue they resemble. Benign STTs greatly outnumber malignant tumors (1). In general, the annual incidence of STTs is 300 per 100,000 population (2). Since malignant tumors can be advanced by the time of presentation, they need to be diagnosed early and treated properly. Detection of STTs is a significant diagnostic challenge because of their morphologic overlap (3). The aim of this study was to determine the utility of fine needle aspiration cytology (FNAC) in diagnosis of STTs and evaluate its correlation with findings of histopathological examination (HPE). In addition, we determined factors that may cause discrepancies between FNAC and histopathology findings.
MATERIALS AND METHODS
This retrospective, record-based study was done on STTs received for FNAC and histopathology examinations at the Department of Pathology, MIMS, Mandya from January 2018 to June 2021. A total of 74 FNAC and biopsy samples were collected between January 2018 and June 2021, from patients suspected of having STTs. The study was approved by the local ethics committee (ethical code: MIMS/IEC/518). All samples were subjected to hematoxylin and eosin (H&E) and May-Grünwald-Giemsa (MGG) staining. Inadequate smears were the ones with 'no' or scanty cells. Non-neoplastic, tumor-like lesions were excluded from the study. In addition, samples taken from cases that were already under treatment for STTs were excluded. Fixation of the excised specimens was done in 10% neutral buffered formalin. After tissue processing, 4-5 µm thick tissue sections were prepared. The sections were stained with H&E. Slides were evaluated under a light microscope.
Data were entered in Microsoft Excel and analyzed using IBM SPSS (version 20).
RESULTS
Of 74 patients suspected of having STTs, 50 (67.56%) were female and 24 (32.43%) were male. The most common anatomical locations of STTs were the upper extremities (35.13%), followed by the head and neck area (21.62%) (Figure 1)

We also found that STTs tend to occur more in the 5th to 6th decades of life. Among FNACs, 71 (95.9%) cases were found to be benign, and 3 (4.05%) cases were malignant. Moreover, most cases were identified as lipoma (Figure 2). For 11 benign tumors, specific tissue diagnosis was not possible; therefore, they were diagnosed as benign spindle cell tumor. Similarly, three malignant tumors were diagnosed as spindle cell sarcomas.

On HPE, we found that 70 (94.6%) cases were benign and 4 (5.4%) cases were malignant (Figure 3). Majority of the cases were of lipoma (68.9%) Among 11 benign spindle cell tumors diagnosed on FNAC, one case was identified as spindle cell lipoma, which was diagnosed so owing to the presence of spindle cell component within the tumor. On histopathology, spindle cell components were seen admixed with adipocytes, which confirmed the diagnosis of spindle cell lipoma (Figure 4). Rest of the benign spindle cell tumors were also diagnosed appropriately (Table 1).
.PNG)
Table 1. Histopathological diagnoses of cases identified as benign spindle cell tumor and spindle cell sarcoma on FNAC


The current study showed that lipoma was the most common benign tumor diagnosis on FNAC and histopathology (67.56% and 68.9%, respectively), while myxofibrosarcoma (spindle cell sarcoma) was the common malignancy among STT (50%).
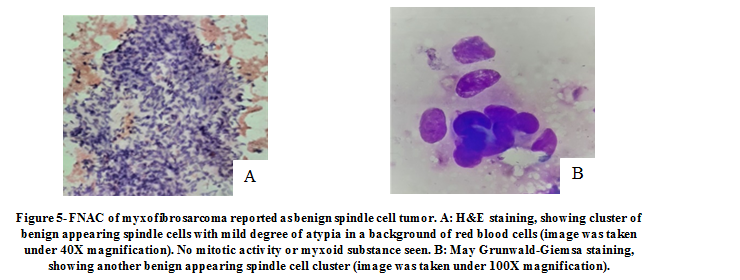
Initially, one tumor was diagnosed as a benign spindle cell tumor on FNAC, and histopathology confirmed the diagnosis to be low grade myxofibrosarcoma. On further review of the respective slides, we deduced that the FNAC diagnosis was based on the presence of only mild atypia among the tumor cells, absence of mitotic figures, and the lack of myxoid substance in the smears, probably because in FNAC, tissue sampling is possible only from a small area of the tumor which, in this case, did not display cytological features of malignancy (Figure 5). In addition, HPE allowed us to sample a larger area of the tissue and showed sheets of oval to spindle cells with hyperchromatic nuclei and sparse mitotic activity in a background of myxoid substance (Figure 6).
DISCUSSION
Soft tissue tumors are the most frequently encountered neoplasms. A thorough clinical examination and appropriate diagnostic procedures are required for proper diagnosis as majority of the cases present as a swelling in any location. The treatment of these tumors can range from a simple excision for benign tumors to an extensive radical surgery if malignant.
In the present study, the majority of cases with STT were female. This is in concordance with the study done by Nagira et al. (4) and in contrast to the study done by Roy et al. (5). Most patients were between 5 and 6 years of age. This finding is in line with the study conducted by Roy et al. (5). We reported upper extremities as the most common site of STT, while Nagira et al. (4) and Bezabih et al. (6) reported lower extremity as the most common site.
In our study, benign tumors were diagnosed more than malignant tumors. On FNAC and histopathology, lipoma was identified as the most common tumor, which is consistent with findings of previous studies (5-7).
In the present study, the sensitivity, specificity, positive predictive value, and accuracy of FNAC in differentiating benign from malignant STTs were 75%, 100%, 100%, and 98.6%, respectively (Table 2). These findings are in line with findings of previous studies (2, 7-12). Indeed, FNAC is able to diagnose most of the benign tumors as benign and differentiate benign from malignant tumors in majority of the cases. The drawback of FNAC is that tissue-based specific morphological diagnosis is not always possible. However, since majority of the benign lesions are managed conservatively, the exact morphological diagnosis is of little significance to the patient and the clinician. The optimal cytological evaluation of soft tissue lesions requires close cooperation between the surgeon and cytopathologist (13). In cases of clinically non-palpable lesions and lesions with poor yield or hemorrhagic aspirate, FNA under radiological guidance proves to be valuable.
Table 2. Statistical comparison of our findings with findings of other studies
The technical issues that arise with FNAC of STTs are as follows:
1. Poor yield: The procedure of aspiration may yield a scant aspirate due to presence of a high collagen or fibrous content within the tumor and improper technique or site of FNAC. For example, sometimes, the connective tissue adjacent to the lesion-like adipose tissue may be aspirated and mistaken for a lipoma.
2. Hemorrhagic yield: In tumors of vascular origin, main content of the aspirate is sparsely cellular; thus, one may miss the tumor cells embedded within a blood clot.
3. If the mass is cystic or necrotic, representative diagnostic areas may be difficult to aspirate adequately (13).
4. Aspirate from cellular areas of benign lesions may display high cellularity and mild to moderately atypical tumor cells, which may lead to an erroneous diagnosis of a malignant STT.
5. Conversely, aspirate from a less cellular area of a malignant tumor, as in the case of low grade myxofibrosarcoma in the present study, may be erroneously diagnosed as a benign tumor.
It is recommended to use FNAC in adjunct with ancillary techniques, such as immunocytochemistry and cytogenetic analysis to increase the diagnostic accuracy. The small number of malignant STTs was a limitation of the present study. Therefore, it is suggested to conduct future studies on a larger sample size.
CONCLUSION
Based on the findings, FNAC is an effective method of rapid diagnosis of STTs. It is an outpatient procedure, which is relatively painless and cheap. The drawbacks of FNAC can be overcome by thorough examination of the patient, expertise in the field of cytopathology, and a general knowledge of the incidences of various STTs. Rarer diagnoses should be made with caution or with an adjunct of other ancillary techniques.
ACKNOWLEDGEMENTS
We would like to acknowledge the support given by the Director, MIMS, Mandya and the Department of Pathology, MIMS, Mandya in conducting this study.
DECLARATIONS
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approvals and consent to participate
This was a retrospective study conducted based on the records maintained in the Department of Pathology. No patient was asked to participate in this study. Ethical approval was obtained by the Institutional Ethics Committee (ethical code: MIMS/IEC/518).
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this article.
Full-Text: (1216 Views)
Soft tissues are non-epithelial extraskeletal tissues of the body. They comprise of skeletal muscles, fat, blood vessels, nerves, and supportive connective tissue. Soft tissues are derived from the mesoderm and their main function is to provide support to various organs. Soft tissue tumors (STTs) are a heterogeneous group of tumors that are classified according to their differentiation into the adult tissue they resemble. Benign STTs greatly outnumber malignant tumors (1). In general, the annual incidence of STTs is 300 per 100,000 population (2). Since malignant tumors can be advanced by the time of presentation, they need to be diagnosed early and treated properly. Detection of STTs is a significant diagnostic challenge because of their morphologic overlap (3). The aim of this study was to determine the utility of fine needle aspiration cytology (FNAC) in diagnosis of STTs and evaluate its correlation with findings of histopathological examination (HPE). In addition, we determined factors that may cause discrepancies between FNAC and histopathology findings.
MATERIALS AND METHODS
This retrospective, record-based study was done on STTs received for FNAC and histopathology examinations at the Department of Pathology, MIMS, Mandya from January 2018 to June 2021. A total of 74 FNAC and biopsy samples were collected between January 2018 and June 2021, from patients suspected of having STTs. The study was approved by the local ethics committee (ethical code: MIMS/IEC/518). All samples were subjected to hematoxylin and eosin (H&E) and May-Grünwald-Giemsa (MGG) staining. Inadequate smears were the ones with 'no' or scanty cells. Non-neoplastic, tumor-like lesions were excluded from the study. In addition, samples taken from cases that were already under treatment for STTs were excluded. Fixation of the excised specimens was done in 10% neutral buffered formalin. After tissue processing, 4-5 µm thick tissue sections were prepared. The sections were stained with H&E. Slides were evaluated under a light microscope.
Data were entered in Microsoft Excel and analyzed using IBM SPSS (version 20).
RESULTS
Of 74 patients suspected of having STTs, 50 (67.56%) were female and 24 (32.43%) were male. The most common anatomical locations of STTs were the upper extremities (35.13%), followed by the head and neck area (21.62%) (Figure 1)

We also found that STTs tend to occur more in the 5th to 6th decades of life. Among FNACs, 71 (95.9%) cases were found to be benign, and 3 (4.05%) cases were malignant. Moreover, most cases were identified as lipoma (Figure 2). For 11 benign tumors, specific tissue diagnosis was not possible; therefore, they were diagnosed as benign spindle cell tumor. Similarly, three malignant tumors were diagnosed as spindle cell sarcomas.

On HPE, we found that 70 (94.6%) cases were benign and 4 (5.4%) cases were malignant (Figure 3). Majority of the cases were of lipoma (68.9%) Among 11 benign spindle cell tumors diagnosed on FNAC, one case was identified as spindle cell lipoma, which was diagnosed so owing to the presence of spindle cell component within the tumor. On histopathology, spindle cell components were seen admixed with adipocytes, which confirmed the diagnosis of spindle cell lipoma (Figure 4). Rest of the benign spindle cell tumors were also diagnosed appropriately (Table 1).
.PNG)
Table 1. Histopathological diagnoses of cases identified as benign spindle cell tumor and spindle cell sarcoma on FNAC
| FNAC diagnosis | Histopathological Diagnosis | Number |
| Benign spindle cell tumor (n=11) | Schwannoma | 4 |
| Hemangioma | 2 | |
| Neurofibroma | 2 | |
| Spindle cell lipoma | 1 | |
| Fibroma | 1 | |
| Low grade myxofibrosarcoma | 1 | |
| Spindle cell sarcoma (n=3) | Dediffferentiated liposarcoma | 1 |
| Myxofibrosarcoma | 1 | |
| MPNST | 1 |



The current study showed that lipoma was the most common benign tumor diagnosis on FNAC and histopathology (67.56% and 68.9%, respectively), while myxofibrosarcoma (spindle cell sarcoma) was the common malignancy among STT (50%).
Initially, one tumor was diagnosed as a benign spindle cell tumor on FNAC, and histopathology confirmed the diagnosis to be low grade myxofibrosarcoma. On further review of the respective slides, we deduced that the FNAC diagnosis was based on the presence of only mild atypia among the tumor cells, absence of mitotic figures, and the lack of myxoid substance in the smears, probably because in FNAC, tissue sampling is possible only from a small area of the tumor which, in this case, did not display cytological features of malignancy (Figure 5). In addition, HPE allowed us to sample a larger area of the tissue and showed sheets of oval to spindle cells with hyperchromatic nuclei and sparse mitotic activity in a background of myxoid substance (Figure 6).
DISCUSSION
Soft tissue tumors are the most frequently encountered neoplasms. A thorough clinical examination and appropriate diagnostic procedures are required for proper diagnosis as majority of the cases present as a swelling in any location. The treatment of these tumors can range from a simple excision for benign tumors to an extensive radical surgery if malignant.
In the present study, the majority of cases with STT were female. This is in concordance with the study done by Nagira et al. (4) and in contrast to the study done by Roy et al. (5). Most patients were between 5 and 6 years of age. This finding is in line with the study conducted by Roy et al. (5). We reported upper extremities as the most common site of STT, while Nagira et al. (4) and Bezabih et al. (6) reported lower extremity as the most common site.
In our study, benign tumors were diagnosed more than malignant tumors. On FNAC and histopathology, lipoma was identified as the most common tumor, which is consistent with findings of previous studies (5-7).
In the present study, the sensitivity, specificity, positive predictive value, and accuracy of FNAC in differentiating benign from malignant STTs were 75%, 100%, 100%, and 98.6%, respectively (Table 2). These findings are in line with findings of previous studies (2, 7-12). Indeed, FNAC is able to diagnose most of the benign tumors as benign and differentiate benign from malignant tumors in majority of the cases. The drawback of FNAC is that tissue-based specific morphological diagnosis is not always possible. However, since majority of the benign lesions are managed conservatively, the exact morphological diagnosis is of little significance to the patient and the clinician. The optimal cytological evaluation of soft tissue lesions requires close cooperation between the surgeon and cytopathologist (13). In cases of clinically non-palpable lesions and lesions with poor yield or hemorrhagic aspirate, FNA under radiological guidance proves to be valuable.
Table 2. Statistical comparison of our findings with findings of other studies
| Variable | Our study (n=74) |
P B Soni et al (n=150) (8) | Pranab Dey et al (n=82) (9) | Veenu Jain et al (n=86) (10) | Vincent et al (n=432) (11) | Parajuli et al (n=50) (2) | Beg et al (n=126) (7) | B Rekhi et al (n=127) (12) |
| Sensitivity | 75% | 70% | 91.5% | 100% | 89.2% | 97.36% | 98.1% | 100% |
| Specificity | 100% | 100% | 92.5% | 98.6% | 89.8% | 66.67% | 96.7% | 87% |
| Positive Predictive value | 100% | 97.9% | 95.5% | 93.3% | 96.1% | - | 97.2% | - |
| Accuracy | 98.6% | 98% | - | 97.7% | - | 80% | - | 98% |
The technical issues that arise with FNAC of STTs are as follows:
1. Poor yield: The procedure of aspiration may yield a scant aspirate due to presence of a high collagen or fibrous content within the tumor and improper technique or site of FNAC. For example, sometimes, the connective tissue adjacent to the lesion-like adipose tissue may be aspirated and mistaken for a lipoma.
2. Hemorrhagic yield: In tumors of vascular origin, main content of the aspirate is sparsely cellular; thus, one may miss the tumor cells embedded within a blood clot.
3. If the mass is cystic or necrotic, representative diagnostic areas may be difficult to aspirate adequately (13).
4. Aspirate from cellular areas of benign lesions may display high cellularity and mild to moderately atypical tumor cells, which may lead to an erroneous diagnosis of a malignant STT.
5. Conversely, aspirate from a less cellular area of a malignant tumor, as in the case of low grade myxofibrosarcoma in the present study, may be erroneously diagnosed as a benign tumor.
It is recommended to use FNAC in adjunct with ancillary techniques, such as immunocytochemistry and cytogenetic analysis to increase the diagnostic accuracy. The small number of malignant STTs was a limitation of the present study. Therefore, it is suggested to conduct future studies on a larger sample size.
CONCLUSION
Based on the findings, FNAC is an effective method of rapid diagnosis of STTs. It is an outpatient procedure, which is relatively painless and cheap. The drawbacks of FNAC can be overcome by thorough examination of the patient, expertise in the field of cytopathology, and a general knowledge of the incidences of various STTs. Rarer diagnoses should be made with caution or with an adjunct of other ancillary techniques.
ACKNOWLEDGEMENTS
We would like to acknowledge the support given by the Director, MIMS, Mandya and the Department of Pathology, MIMS, Mandya in conducting this study.
DECLARATIONS
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approvals and consent to participate
This was a retrospective study conducted based on the records maintained in the Department of Pathology. No patient was asked to participate in this study. Ethical approval was obtained by the Institutional Ethics Committee (ethical code: MIMS/IEC/518).
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this article.
Research Article: Research Article |
Subject:
Pathology
Received: 2021/12/17 | Accepted: 2022/02/12 | Published: 2022/05/14 | ePublished: 2022/05/14
Received: 2021/12/17 | Accepted: 2022/02/12 | Published: 2022/05/14 | ePublished: 2022/05/14
References
1. Weiss, Sharon W. Enzinger and Weiss's Soft Tissue Tumors. 7th ed. St. Louis :Mosby, 2020. [View at Publisher] [Google Scholar]
2. Parajuli S, Lakhey M. Efficacy of fine needle aspiration cytology in diagnosing soft tissue tumors. Journal of Pathology of Nepal. 2012 Sep 25;2(4):305-8. [DOI:10.3126/jpn.v2i4.6884] [Google Scholar]
3. Chang AE, Rosenberg SA, Glastein EJ, Antman KH: Sarcomas of soft tissues. In Cancer: Principles and Practice of Oncology 3rd edition. Edited by: Vita VD, Hellman S, Rosenberg SA. Philaldelphia: JB Lippincott;1989:1345-1398
4. Nagira K, Yamamoto T, Akisue T, Marui T, Hitora T, Nakatani T, et al. Reliability of fine‐needle aspiration biopsy in the initial diagnosis of soft‐tissue lesions. Diagnostic cytopathology. 2002; 27(6): 354-61. [View at Publisher] [DOI:10.1002/dc.10200] [PubMed] [Google Scholar]
5. Roy S, Manna AK, Pathak S, Guha D. Evaluation of fine needle aspiration cytology and its correlation with histopathological findings in soft tissue tumours. Journal of cytology. 2007; 24(1): 37. [View at Publisher] [DOI:10.4103/0970-9371.42089] [Google Scholar]
6. Bezabih M. Cytological diagnosis of soft tissue tumours. Cytopathology. 2001; 12(3): 177-83. [View at Publisher] [DOI:10.1046/j.1365-2303.2001.00317.x] [Google Scholar]
7. Beg S, Vasenwala SM, Haider N, Ahmad SS, Maheshwari V, Khan MA. A comparison of cytological and histopathological findings and role of immunostains in the diagnosis of soft tissue tumors. Journal of Cytology/Indian Academy of Cytologists. 2012 Apr;29(2):125 [View at Publisher] [DOI:10.4103/0970-9371.97154] [PubMed] [Google Scholar]
8. Soni PB, Verma AK, Chandoke RK, Nigam JS. A prospective study of soft tissue tumors histocytopathology correlation. Pathology research international. 2014;2014. [View at Publisher] [DOI:10.1155/2014/678628] [PubMed] [Google Scholar]
9. Dey P, Mallik MK, Gupta SK, Vasishta RK. Role of fine needle aspiration cytology in the diagnosis of soft tissue tumours and tumour‐like lesions. Cytopathology. 2004; 15(1): 32-7. [View at Publisher] [DOI:10.1046/j.0956-5507.2003.00102.x] [PubMed] [Google Scholar]
10. Jain V, Agarwal T. Role of FNAC in soft tissue tumors and its histopathological correlation. International Surgery Journal. 2017 Jul 24;4(8):2632-6. [View at Publisher] [DOI:10.18203/2349-2902.isj20173402] [Google Scholar]
11. Ng VY, Thomas K, Crist M, Wakely PE, Mayerson J. Fine needle aspiration for clinical triage of extremity soft tissue masses. Clinical Orthopaedics and Related Research®. 2010; 468(4): 1120-8. [View at Publisher] [DOI:10.1007/s11999-009-1100-7] [PubMed] [Google Scholar]
12. Rekhi B, Gorad BD, Kakade AC, Chinoy RF. Scope of FNAC in the diagnosis of soft tissue tumors-a study from a tertiary cancer referral center in India. Cytojournal. 2007;4:20. [DOI:10.1186/1742-6413-4-20] [PubMed] [Google Scholar]
13. Domanski HA. Fine‐needle aspiration cytology of soft tissue lesions: diagnostic challenges. Diagnostic cytopathology. 2007; 35(12): 768-73. [View at Publisher] [DOI:10.1002/dc.20765] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







